Abstract
Transarterial radioembolization (TARE) with yttrium-90 microspheres has become widely utilized in managing hepatocellular carcinoma (HCC). The utility of TARE is expanding with new insights through experiences from real-world practice and clinical trials, and recently published data suggest that TARE in combination with sorafenib may improve the overall survival in selected patients. Here, we report a case of advanced stage HCC that was successfully treated with TARE and sorafenib. The patient achieved complete response (CR) at 12 months after the initial treatment with TARE and sorafenib, followed by additional transarterial chemoembolization and proton beam therapy for local tumor recurrence at 19-month post-TARE. The patient was followed up every 3 months thereafter and still achieved CR both biochemically and radiologically for the following 12 months. A combination strategy of TARE and systemic therapy may be a useful alternative treatment option for selected patients with advanced stage HCC.
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver and the second leading cause of cancer-related mortality worldwide.1,2 Based on the Barcelona Clinic Liver Cancer (BCLC) staging system, stage 0 or A patients with very early- or early-stage disease are eligible for curative therapies including ablative therapies, surgical resection, or liver transplant.3 However, significantly few patients are eligible for these curative options due to the presence of advanced disease upon presentation. For intermediate or advanced stage HCCs, standard of care includes transarterial chemoembolization (TACE) or systemic therapy.3 Recently, transarterial radioembolization (TARE) with yttrium-90 (90Y) microspheres has become an increasingly popular treatment option.4 TARE is performed by image-guided deposition of radiated 90Y microspheres to the tumor through the hepatic artery, which is the most common source of perfusion for HCC. TARE with 90Y microsphere has emerged as an alternative treatment to TACE, and this treatment option has comparable survival rate with TACE but results in superior quality of life compared to TACE. Moreover, this treatment is applicable for advanced stage HCC with reduced risk of ischemic hepatitis considering that arterial occlusion is not commonly observed in this treatment.5–7 For patients with advanced HCC, sorafenib is the first-line therapeutic option; however, sorafenib has a more modest survival benefit compared to placebos.8–10 Some in vitro and in vivo studies had investigated the possible synergistic effect between TARE and sorafenib therapy.11,12 Moreover, according to a palliative sub-study of the Sorafenib and Micro-therapy Guided by Primovist Enhanced magnetic resonance imaging (MRI) in Patients With Inoperable Liver Cancer (SORAMIC trial, NCT01126645), the interim safety analysis reassures that 90Y radioembolization as a sequential approach followed by an escalation scheme for sorafenib does not lead to increased toxicity.13 Herein, we describe the case of an advanced stage HCC patient who showed a favorable response following a combination treatment with TARE and sorafenib.
A 56-year-old male patient presented with multinodular liver masses incidentally detected on screening ultrasonography at another hospital. The patient was diagnosed with chronic hepatitis B 20 years prior, but he was not under regular HCC surveillance. The patient had consumed 1 bottle of soju (Korean distilled liquor with an alcohol content of 15–20%), 3 times a week, but abstained from alcohol since he was diagnosed with HCC. He had no associated symptoms, and there were no remarkable findings on physical examination. Initial laboratory findings were as follows: white blood cell, 4,200/μL; hemoglobin, 15.0 g/dL; platelet, 137,000/μL; total bilirubin, 0.8 mg/dL; albumin, 4.1 g/dL; international normalized ratio, 1.08; aspartate aminotransferase, 92 IU/L; alanine aminotransferase, 51 IU/L; and serum hepatitis B virus DNA 7,787,219 IU/mL. Alpha-fetoprotein (AFP) level was 33,996 ng/mL, and protein induced by vitamin K absence or antagonist-II (PIVKA-II) level was 51 mAU/mL. The liver function was preserved (Child-Pugh score, 5).
Initial contrast-enhanced multiphasic liver computed tomography (CT) revealed a 6-cm-sized liver mass in segment (S) 5/8 with multiple intrahepatic satellite nodules and enlarged lymph nodes in the common hepatic artery area and portacaval space. The liver mass showed typical radiological features of HCC-hyperenhancement on the arterial phase and washout on portal venous and delayed phases. Liver dynamic MRI revealed multifocal tumor thrombosis involving S5/S8 portal vein branches and middle hepatic vein (Fig. 1A–D). Chest CT revealed multiple pulmonary metastases in both lungs (Fig. 1E).
The patient was diagnosed with an advanced stage HCC (BCLC stage C, modified Union for International Cancer Control T4N1M1, stage IVB) without liver biopsy since typical features of HCC in imaging results were observed.14 The patient was treated with 90Y TARE for the local control of intrahepatic lesions, followed by sorafenib for the systemic control of the tumor. For underlying chronic hepatitis B and cirrhosis, entecavir 0.5 mg was administered. TARE was performed using TheraSphere® glass microspheres (BTG PLC, London, UK); the total infused radiation activity was 14 gigabecquerel (GBq) with 3 GBq for S4, 3 GBq for S5, and 8 GBq for S5/S8. The dose was calculated using the Medical Internal Radiation Dose method, as recommended by the manufacturer. No adverse events or clinical signs of hepatic decompensation were observed after the procedure. The patient started to take sorafenib 400 mg twice daily 1-month post-TARE. However, sorafenib dose was reduced to 400 mg once daily since grade 2 adverse events with hand-foot-skin reaction (HFSR), which developed 2 weeks after beginning sorafenib, were observed.
At 3-month post-TARE, partial response was achieved according to the modified Response Evaluation Criteria In Solid Tumors (RECIST) criteria.15 Multifocal infiltrative lesions in the right hepatic lobe significantly decreased in extent with reduced arterial enhancement. Furthermore, metastatic lung nodules also decreased in number and size. AFP level decreased greater than 10-fold at 1,290 ng/mL.
At 12-month post-TARE, complete response (CR) was achieved with the absence of arterial enhancement on target liver lesion, and metastatic nodules in both lungs (Fig. 2) and portal/hepatic vein thrombosis were already not observed. Serum PIVKA-II level was within the normal range at 26 mAU/mL; however, serum AFP level remained slightly elevated at 249 ng/mL. After achieving radiological CR, the patient refused further sorafenib treatment considering that grade 2 adverse events with HFSR were observed during sorafenib treatment.
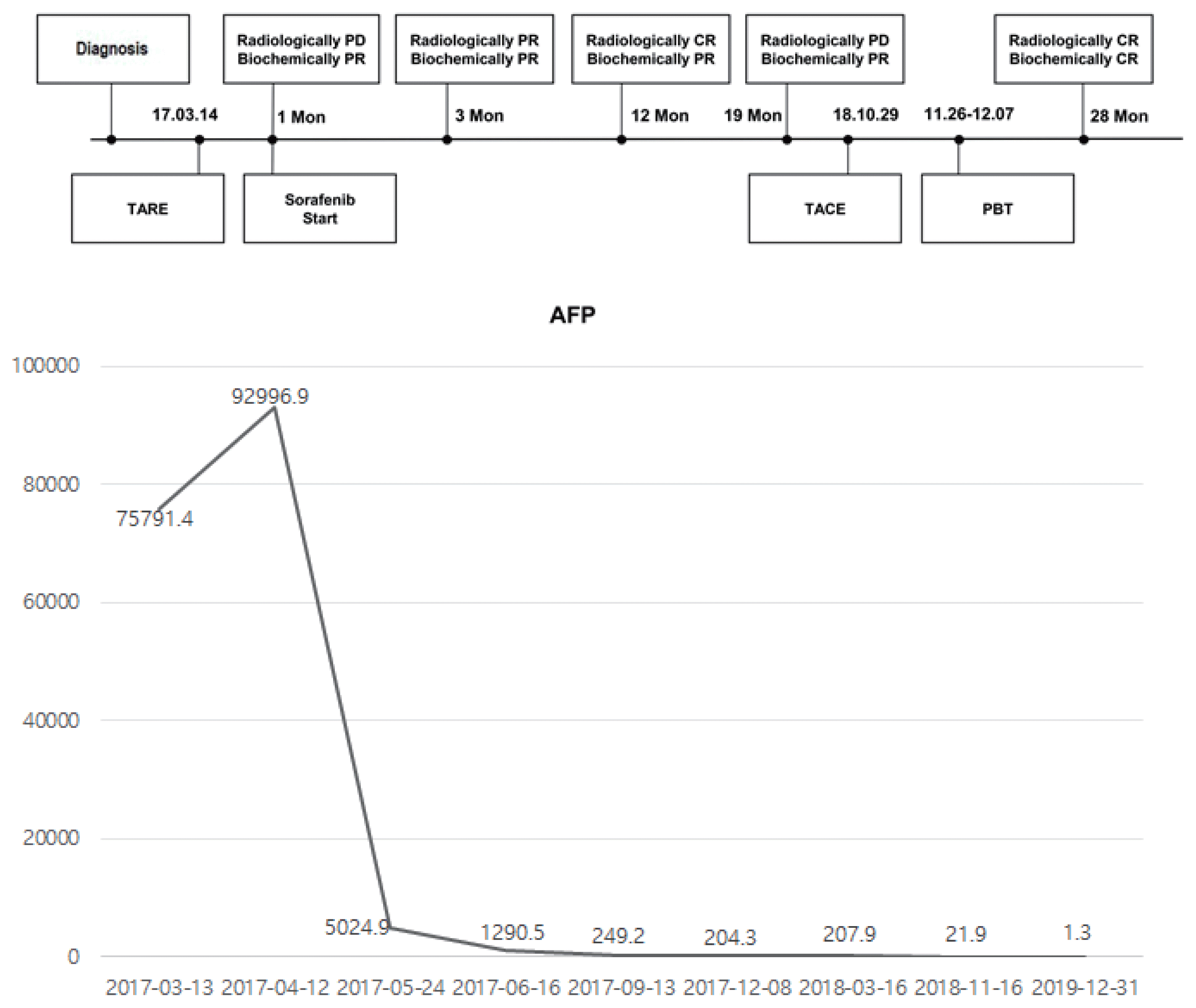
At 19-month post-TARE, an arterial enhancing nodule measuring up to 8 mm was observed in the subcapsular area of S8/S4, which displayed diffusion restriction in liver dynamic MRI and an uptake of maximum standardized uptake value (SUVmax) 5.3 in positron emission tomography-CT, consistent with local tumor recurrence with suspicious tumor thrombosis at the portal vein of S4 (P4) (Fig. 3). Subsequently, additional TACE was performed for the viable tumor at S8/S4 followed by a consolidation proton beam therapy of 6.6 cobalt gray equivalent on S4 and P4. AFP level was within the normal range 2 months after TACE and proton beam therapy. The patient was followed up every 3 months thereafter and still achieved CR both biochemically and radiologically for the following 12 months. Clinical course is described in Fig. 4 based on treatment modality, treatment response, and change in AFP.
Here, we present an interesting case of advanced stage HCC that was successfully treated with TARE and sorafenib. The patient achieved CR through initial treatment with TARE and sorafenib, followed by additional TACE and proton beam therapy for local tumor recurrence.
According to the current practice guidelines, systemic therapy with a multi-tyrosine kinase inhibitor (TKI) (i.e., sorafenib or lenvatinib) is recommended as the first-line therapeutic option for advanced stage HCC.3,16 However, only a few proportion of patients with advanced stage HCC benefit from TKIs, with modest survival gain of only 2–3 months.8–10 Some studies, including five large randomized trials, were conducted to evaluate the effectiveness of TACE and sorafenib combination therapy. These studies confirmed that the overall survival rate was lower in this combination therapy than in sorafenib therapy alone.17 TARE is better tolerated than TACE, which may allow a higher dose and longer length of therapy with sorafenib.18 Additionally, some in vitro and in vivo studies found that sorafenib may be synergistic with TARE considering the antiangiogenic effect of sorafenib, which allowed the tumor vessels to deliver oxygen more efficiently to the core of HCC tumors, possibly enhancing the effect of radiation-induced tumor regression.11,12
On the contrary to theoretical rationale, the first large randomized controlled trial, SORAMIC, failed to demonstrate a higher survival benefit of TARE and sorafenib combination therapy than sorafenib therapy alone for the palliative treatment of unresectable HCC. However, a possible overall survival benefit was observed in some subgroups including younger patients aged <65 years, those with nonalcoholic cirrhosis, and those without cirrhosis.19 The current patient was a good candidate for 90Y TARE plus sorafenib combination therapy considering his young age and preserved liver function.
Another phase 3 prospective study, the TheraSphere in the Treatment of Patients with Unresectable Hepatocellular Carcinoma (STOP-HCC trial, NCT01556490), that comprises a larger sample size that aimed to detect a difference in the overall survival between the combined therapy and sorafenib alone therapy in unresectable HCC is ongoing, and preliminary results of the two first interim analyses support the continuation of the study.20
Safety analyses in the SORAMIC study reported higher incidence of grade 3–4 adverse events in the combination therapy group than in the sorafenib only group.13 Grade 2 adverse events with HFSR were developed in this case, and the patient refused further sorafenib treatment after achieving radiological CR.
In conclusion, we described a case of advanced stage HCC that was successfully treated with TARE and sorafenib without severe adverse events. Thus, a combination strategy of TARE and systemic therapy may be a useful alternative treatment option for selected patients with advanced stage HCC.
ACKNOWLEDGMENTS
The present case report was approved by the Institutional Review Boards (IRBs) of the Samsung Medical Center (IRB No.2020-03-047). Written informed consent was obtained from the patient for publication of this case report. The authors acknowledge grant support of the National Research Foundation of Korea (NRF) (2016R1C1B2015463, W.K).
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
2. Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol (Pozn). 2018; 22:141–150.
3. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.
4. Salem R, Gabr A, Riaz A, Mora R, Ali R, Abecassis M, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018; 68:1429–1440.
5. Kim DY, Han KH. Transarterial chemoembolization versus transarterial radioembolization in hepatocellular carcinoma: optimization of selecting treatment modality. Hepatol Int. 2016; 10:883–892.
6. Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Dis. 2014; 34:435–443.
7. Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013; 36:714–723.
8. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017; 67:999–1008.
9. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
10. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
11. Plastaras JP, Kim SH, Liu YY, Dicker DT, Dorsey JF, McDonough J, et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007; 67:9443–9454.
12. Pracht M, Edeline J, Lepareur N, Lenoir L, Ardisson V, Clement B, et al. In vitro demonstration of synergy/additivity between (188) rhenium and sorafenib on hepatoma lines: preliminary results. Anticancer Res. 2013; 33:3871–3877.
13. Ricke J, Bulla K, Kolligs F, Peck-Radosavljevic M, Reimer P, Sangro B, et al. Safety and toxicity of radioembolization plus Sorafenib in advanced hepatocellular carcinoma: analysis of the European multicentre trial SORAMIC. Liver Int. 2015; 35:620–626.
14. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Korean J Radiol. 2019; 20:1042–1113.
15. European Association for the Study of the Liver, Clinical practice guidelines panel, Wendon J, Panel M, Cordoba J, Dhawan A, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017; 66:1047–1081.
16. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.
17. Kudo M, Arizumi T. Transarterial chemoembolization in combination with a Molecular Targeted Agent: Lessons Learned from Negative Trials (Post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2). Oncology. 2017; 93(Suppl 1):127–134.
18. Forner A, Da Fonseca LG, Díaz-González Á, Sanduzzi-Zamparelli M, Reig M, Bruix J. Controversies in the management of hepatocellular carcinoma. JHEP Reps. 2019; 1:17–29.
19. Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. 2019; 71:1164–1174.
20. Chauhan N, Bukovcan J, Boucher E, Cosgrove D, Edeline J, Hamilton B, et al. Intra-Arterial TheraSphere Yttrium-90 Glass Microspheres in the treatment of patients with unresectable hepatocellular carcinoma: protocol for the STOP-HCC Phase 3 randomized controlled trial. JMIR Res Protoc. 2018; 7:e11234.
Figure 1
Initial liver dynamic magnetic resonance image findings. Multinodular mass showed enhancement on the arterial phase (A) and washout on the portal phase (B–D). The tumor thrombosis involved in the portal vein branches and mid-hepatic vein (arrows). (E) Initial chest computed tomography findings. Multiple pulmonary metastases in both lungs were observed.
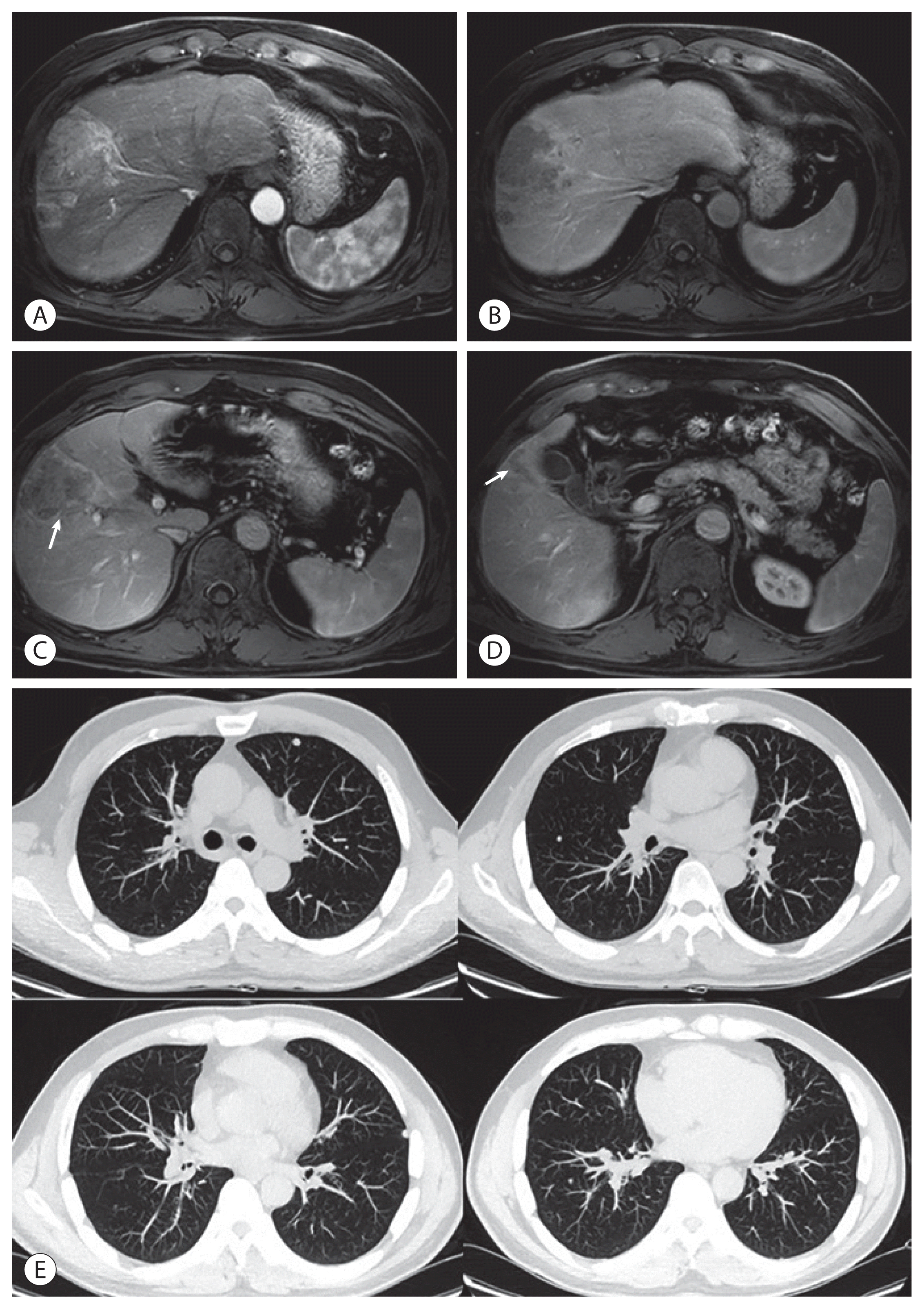
Figure 2
Follow-up liver dynamic computed tomography (CT) findings at 1-month (A), 3-month (B), 6-month (C), and 12-month (D) post-transarterial radioembolization (TARE). Follow-up chest CT findings at 1-month (E), 3-month (F), 6-month (G), and 12-month (H) post-TARE.
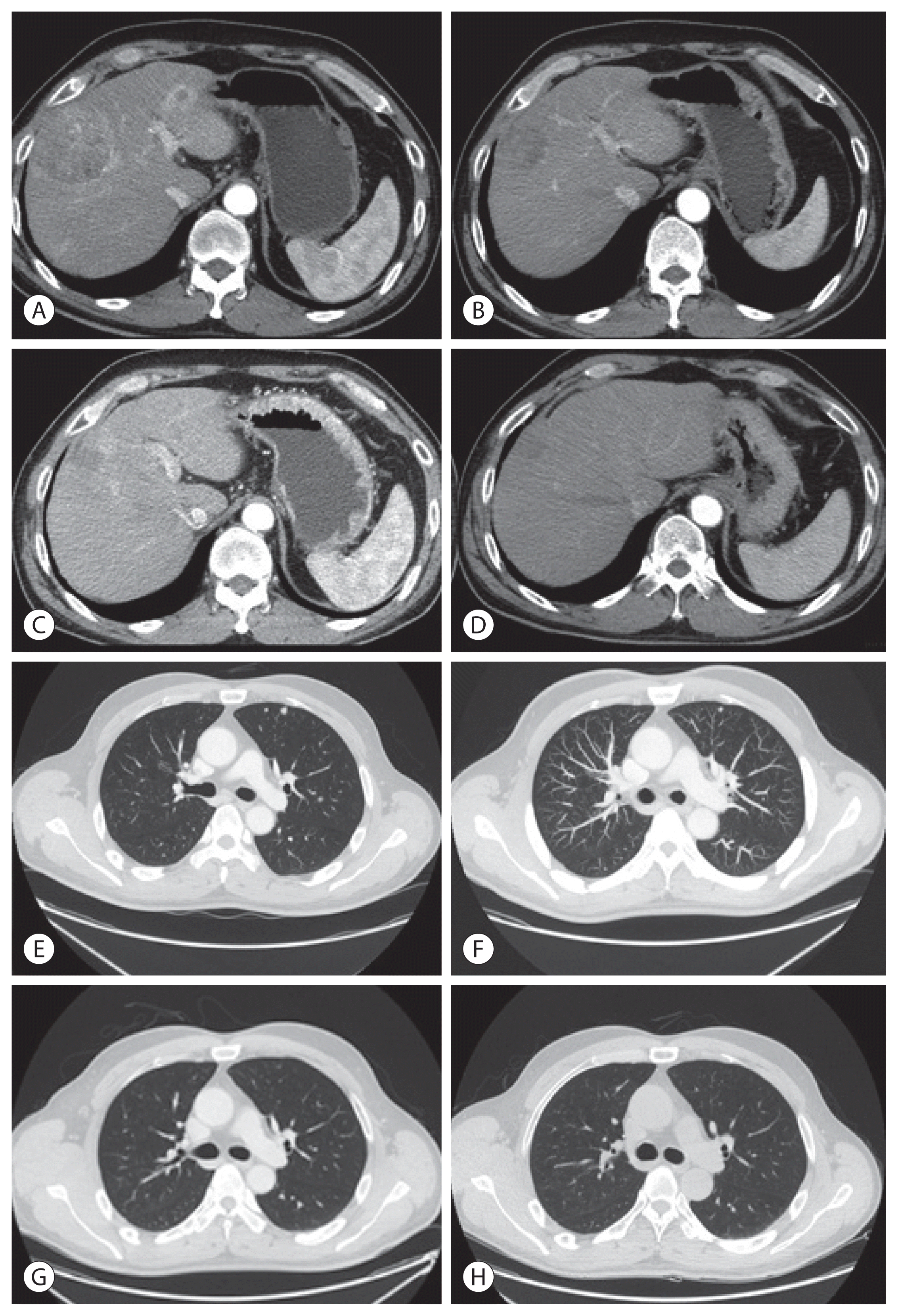
Figure 3
Follow-up liver dynamic magnetic resonance imaging at 19-month post-transarterial radioembolization showing a subcentimeter-sized enhancing viable tumor at the top of segment 8/4 lesion on the arterial phase (A) with diffusion restriction (B). A focal hypermetabolic lesion (maximum standardized uptake value [SUVmax]=5.3) at the corresponding lesion in positron emission tomography-computed tomography (C). Hepatic angiography. Multiple punctate tumor stainings at segment 8/4 were observed (D).

Figure 4
Clinical progress including treatment modalities, treatment response, and change in tumor markers (bottom) during the follow-up period. Mon, months; TARE, transarterial radioembolization; TACE, transarterial chemoembolization; PBT, proton beam therapy; PD, progressive disease; PR, partial response; CR, complete response; AFP, alpha-fetoprotein.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download