Abstract
Transcatheter arterial chemoembolization (TACE) is a useful palliative therapeutic modality for hepatocellular carcinoma (HCC). Postembolization syndromes, such as fever, abdominal pain, and elevated liver enzyme levels are commonly known complications of TACE. One post-TACE pulmonary complication, lipiodol pneumonitis, is rarely reported. Lipiodol pneumonitis after TACE appears to be associated with chemical injury due to accidental perfusion of lipiodol to the lung vasculature, promoted by arteriovenous shunts within the hypervascular HCC. Here, we report a 42-year-old man with unresectable HCC and hepatic vein thrombosis. The patient was initially treated with TACE. The following day after TACE, acute respiratory symptoms such as dyspnea and cough developed with decreased oxygen saturation. Chest X-ray and computed tomography showed multiple patches and diffuse ground-glass opacities in both lung fields, suggesting of lipiodol pneumonitis. The patient’s condition and radiologic abnormalities subsequently improved after 2 weeks of conservative treatment alone.
Hepatocellular carcinoma (HCC), the most common primary liver cancer, is a major cause of cancer-related death in the world. HCC accounts for about 85% of primary liver cancer in Korea. In Asia, where hepatitis B virus infection rates are high, the disease is of greater importance. HCC is often found in a considerably advanced state at the time of diagnosis. In addition, chronic hepatitis and cirrhosis often accompany HCC, and it is often difficult to perform curative treatment. The treatment for unresectable HCC is hepatic arterial chemotherapy and transarterial embolization. Transcatheter arterial chemoembolization (TACE) using a mixture of lipiodol and anticancer agents has been actively used to treat unresectable HCC. Hepatic arterial chemoembolization involves lipiodol, gelatin sponge (gelfoam) and an anticancer agent injected into the hepatic artery to induce necrosis of cancer cells through stagnation of the anticancer drug and microvascular embolization. Complications of hepatic arterial chemoembolization include symptoms of postembolization syndrome, such as fever, abdominal pain, nausea, vomiting, leukocytosis, and elevated liver enzyme levels. In addition, it is known that ascites, hepatitis, hepatic encephalopathy, ischemic cholecystitis, rupture of HCC, hepatic abscess, and severe hepatic failure may occur.1 One post-TACE pulmonary complication, lipiodol pneumonitis, is rarely reported. Here, we report a rare case of lipiodol pneumonitis after TACE for unresectable HCC.
A 42-year-old man presented to our hospital with abdominal pain. Further evaluation revealed the presence of an approximately 7.5 cm hepatic mass in the posterior section of the right hemiliver. The patient has a medical history of diabetes mellitus and chronic hepatitis B. The patient had been drinking about 38 mL of alcohol (half a bottle of soju) once a week for the past 20 years. There were no specific findings in his family history. On physical examination, the patient had tenderness in the right upper abdomen. Peripheral blood tests showed a white blood cell count of 4,860 cells/μL, hemoglobin level of 11.7 g/dL, and platelet count of 304,000 cells/μL. Serum biochemical tests showed total protein of 7.1 g/dL, albumin of 3.4 g/dL, aspartate aminotransferase of 25 U/L, alanine aminotransferase of 17 U/L, total bilirubin of 0.43 mg/dL with direct bilirubin of 0.22 mg/dL, alkaline phosphate of 235 U/L, and gamma-glutamyl transpeptidase of 82 U/L. Blood coagulation tests showed a prothrombin time international normalized ratio of 1.32. Tumor marker testing showed alpha-fetoprotein at 80.3 ng/mL, protein induced by vitamin K absence/antagonist-II at 8,285 mAU/mL, and carbohydrate antigen 19-9 at 10.4 U/mL. Viral markers of hepatitis B and C were positive for hepatitis B surface antigen, negative for hepatitis B surface antibody, negative for hepatitis B e antigen, positive for hepatitis B e antibody, and negative for hepatitis C antibody and had HBV DNA of 84 IU/mL.
The initial chest X-ray showed no specific findings. On computed tomography (CT), there was a 7.5 cm infiltrative mass showing enhancement in the arterial phase and washout in the delayed phase in the right hemiliver. Magnetic resonance imaging (MRI) using a hepatocyte-specific contrast agent (Primovist, Bayer; Leverkusen, Germany) revealed a 7.5 cm sized infiltrative mass with low T1 signal intensity, moderate T2 signal intensity, contrast enhancement in the arterial phase, and washout in the portal and hepatobiliary phases in the right hemiliver (Fig. 1). Right hepatic vein thrombosis, thrombosis of the inferior vena cava (IVC), right ventricle thrombosis, and spleen enlargement were observed.
The patient had chronic hepatitis B virus infection, considered to be a risk factor for HCC, and could be diagnosed as HCC because of the typical image findings by dynamic CT and MRI. Chest CT and bone scan were performed for cancer staging. On the chest CT, several small nodules were observed in the periphery of both lungs and were suspected to be lung metastases. The liver function grade was classified as Child-Pugh Class A (6 points), Eastern Cooperative Oncology Group (ECOG) Performance Status Grade was 0, and the cancer stage was classified as modified Union for International Cancer Control stage IV B, Barcelona Clinic Liver Cancer (BCLC) stage C. The combination of TACE and radiation therapy (RT) was planned as treatment for this patient to control the primary site. The patient underwent selective hepatic artery chemoembolization with 50 mg of adriamycin and 17 mL of lipiodol (Fig. 2). The day after the procedure, acute respiratory symptoms such as dyspnea and dry cough developed. There was a decrease in oxygen saturation down to 80%. On physical examination, there was a decrease in respiratory sounds in both lungs and some wheezing sounds were heard. The chest CT (Fig. 3) and X-ray (Fig. 4A) showed multifocal lipiodol accumulations, multiple patches, and diffuse ground-glass opacities in both lung fields, suggestive of lipiodol pneumonitis. Empirical antibiotics (piperacillin and tazobactam) and high-flow oxygen were supplied according to presumed pneumonitis. Blood culture and sputum cultures did not show microorganisms, and it was judged to be pneumonitis due to chemical factors rather than bacterial pneumonia because pyogenic sputum and high fever were not observed. After 7 days of symptomatic treatment, symptoms of respiratory distress gradually improved, and a chest X-ray showed an improvement in infiltration (Fig. 4B-D). On the 15th day of hospitalization, there were no symptoms of dyspnea. Arterial blood gas analysis and a chest X-ray showed no abnormal findings. The patient was discharged after RT for the liver mass and IVC and right atrial thrombi. He received additional RT for a newly detected abdominal pericaval metastatic lymph node. He was then treated with sorafenib due to lung metastasis but stopped sorafenib 43 days later due to pneumonia development, worsening ECOG from 0 to 2, and progression of lung metastasis. He died eight months after diagnosis with advanced HCC under the best supportive and hospice care.
This case was a rare case of lipiodol pneumonitis after TACE in HCC with hepatic vein involvement. In the 1980s, hepatic artery chemoembolization was developed with lipiodol in combination with anticancer drugs using the property of selective accumulation in liver cancer tissue, and TACE has been actively used as non-surgical therapy for HCC. Embolization has a direct effect on ischemic necrosis of the tumor. In addition, by blocking blood flow, it also plays a role of maintaining a high concentration of the anticancer drug in the tumor for a long time and suppressing the extracellular efflux of the drug, thereby reducing side effects. TACE is effective in the treatment of HCC, but unintended complications of chemoembolization have been reported [1].
The complications of hepatic arterial chemoembolization include postembolization syndrome, with symptoms such as fever, abdominal pain, nausea, vomiting, leukocytosis, and elevated liver enzyme levels. In addition, it is known that ascites, hepatitis, hepatic encephalopathy, ischemic cholecystitis, rupture of HCC, hepatic abscess, and severe hepatic failure may occur. Among the respiratory complications, there was a case in which acute respiratory failure occurred 30 minutes after injection of a mixture of lipiodol and adriamycin into the hepatic artery [2]. There have been several cases of pulmonary embolism and cerebral embolism simultaneously in liver cancer patients after hepatic arterial chemoembolization [3]. The incidence of pulmonary oil embolism was reported as 0.05-2.3% [4,5]. However, lipiodol-induced pneumonitis following TACE is very rare [6,7].
In this case, there are many things that can be considered as a cause of lipiodol pneumonitis. The lipiodol itself may be an induction factor. Lipiodol, an ethyl ester of fatty acids from poppyseed oil, is a contrast agent used in lymphangiography. Since its function as a carrier of anticancer agents became known in the early 1980s, it became widely used in TACE. Lipiodol is mixed with an anticancer agent to form an emulsion and then injected into the artery, where it gradually releases the anticancer agent, thereby maintaining high concentrations of the anticancer agent in the tumor tissue. The injection of lipiodol into the hepatic artery is selectively distributed in the tumor due to the absence of Kupffer cells to clear lipiodol in the tumor tissue, the siphon effect due to tumor hypervascularity, and the high mucoid properties of lipiodol. It is present in blood vessels for a long period of time, from several weeks to a year [8]. These properties of lipiodol may be involved in the development of lipiodol pneumonitis. Although the mechanism of lipiodol induced pneumonitis following TACE is still unclear, the most probable hypothesis for symptomatic lipiodol pneumonitis is pulmonary capillary leakage and edema caused by enzymatic degradation of lipiodol by lipases and formation of toxic unbound free fatty acids [9]. There is also a report suggesting that pneumonitis caused by lipiodol containing radioactive iodine is due to a bioreaction involving an allergic phenomenon and radioactive-induced lesions [10]. The evidence supporting this finding is that polymorphonuclear neutrophils or cluster of differntiation 8+ T-cells are predominant in bronchoalveolar lavage fluid, that alveolar fibrosis or endothelial damage is common in interstitial pneumonia, and that clinical features and imaging findings are similar to radiation pneumonitis. However, it has been reported that it takes several days to several weeks to cause pneumonitis due to this mechanism, which seems unlikely to be the direct cause in this patient.
The amount of lipiodol used in the procedure is also one of the most important induction factors, especially when more than 20 mL of lipiodol is infused [11]. It has been previously reported that the degree of tissue damage caused by the injection of lipiodol into a normal rabbit hepatic artery is proportional to the amount of lipiodol [12]. In this case, an emulsion of 50 mg of adriamycin and 15 mL of lipiodol was prepared, and the mixture was administered intra-arterially to the right hepatic artery followed by gelfoam particles. The left hepatic artery also received an injection of 2 mL of lipiodol. As a result, a total of 17 (almost 20) mL of lipiodol was injected in this patients.
It is also possible that adriamycin itself may have been the causative agent of lipiodol pneumonitis. In this case, it is possible that adriamycin was injected into the pulmonary parenchyma and pleural membrane through the arteriovenous (AV) shunt in the HCC, resulting in pulmonary edema and pneumonitis. However, the possibility of adriamycin-induced pneumonitis is unlikely because there are few instances that would result in a major problem for adriamycin-induced pulmonary complications used in the TACE procedure. In addition, it is more reasonable to consider lipiodol pneumonitis because of the patient’s relatively rapid clinical improvement by conservative treatment alone as well as the definite evidence of lipiodol deposition on the chest CT. Furthermore, radiologic findings such as diffuse interstitial space and interlobular septal hypertrophy provide a basis of chemical pneumonitis caused by lipiodol rather than bacterial pneumonia. However, in this patient, antibiotics were also used because of clinical features such as fever and leukocytosis, and so bacterial pneumonia cannot be excluded at the start of treatment.
The presence of an AV shunt may be a risk factor for lipiodol pneumonitis. If AV shunting is detected before the procedure, the risk of lipiodol pneumonitis increases [13]. In patients with liver cirrhosis and liver cancer, hepatic and pulmonary AV shunting may occur in association with hepatic venous invasion. When hepatic arterial embolization is performed, these emboli can enter the systemic circulation through a short circuit and cause embolism in various organs. However, it has also been reported that pulmonary embolism due to lipiodol or respiratory failure can present even if there is no AV shunt [14]. In this case, the AV shunt identified on the angiography is likely to have accelerated the incidence of lipiodol pneumonitis. This is mainly thought to occur when the injected material migrates into the venous system through AV shunts located in the hypervascular HCC tumors. If AV shunting into branches of the hepatic vein is present, the emulsion and gelfoam will reach the hepatic vein and the pulmonary circulation, producing unwanted side-effects in the lungs [15]. This suggests that lipiodol deposited in the lungs after the procedure may cause edema and pulmonary disorder through inflammatory reaction and cause pneumonitis. In the case of lipiodol pneumonitis, if the size of the HCC is large with invasion into the liver vessel (like the patient in the present case), inflammation is likely due to deposition of lipiodol in the lung through the AV circuit of the liver and lung. In general, It has been reported that if AV shunting is detected by angiography, suspension of subsequent chemoembolization should be considered [16]. In addition, AV shunting may be a problem in HCC patients treated with TACE because anticancer agents can pass through the shunts and cause systemic toxicity, as well as decrease the anticancer effects against the tumor [17]. Whole-body scintigraphy using hepatic arterial injection of albumin Tc99-labeled macro-aggregates can be performed prior to TACE to calculate the degree of AV shunting. Such a process reduces the risk of procedures. In certain cases, this precautionary measure can prevent pulmonary complications, especially when there is hepatic vein involvement or when injection of a large amount of lipiodol is expected. If significant shunting is 20% or more, it is preferable to suspend the chemoembolization [18]. If AV shunting is identified but chemoembolization is considered essential, it is advisable to use larger diameter beads and adjust the drug dosage [18]. Another alternative is the occlusion of the shunt at the early stages of the procedure using balloon insertion or other embolization materials (such as gelfoam particles, coils, polyvinyl alcohol, and empyrospher) [19]. The Amplatzer Vascular Plug can be effective in cases of large AV shunts and high blood flow [20]. This is because of its advantages in ease of delivery and less migration or reposition.
In severe cases, pulmonary embolism may cause a decrease in pulmonary surfactant, resulting in atelectasis or pneumonitis [11]. This is also possible when symptoms are rapidly progressing. However, in this case, there was no thrombus or mass on the pulmonary artery, and it clinically improved rapidly after the treatment. Therefore, the diagnosis of pneumonitis was thought to be more appropriate than embolism because the deposition of lipiodol on the chest CT was definitively observed.
It is also known that chemoembolization via the inferior phrenic artery (IPA) is a risk factor for lipiodol pneumonitis after TACE [9]. This is because the collateral circulation between the inferior phrenic artery and the pulmonary artery branch often leads to chemoembolic agents flowing into the lungs. Therefore, in case of TACE via the IPA, superselective IPA angiography and occlusion for shunts to pulmonary vessels is necessary to prevent pulmonary complications.
We have presented a rare case of lipiodol pneumonitis secondary to TACE for HCC. In this case, it is suspected that the progression of lipiodol pneumonitis was induced by injected lipiodol migrating into the venous system through AV shunts located in the hypervascular HCC tumors. Furthermore, the patient had suspected lung metastasis, which can be presumed to have accelerated the progression of pneumonitis. However, the mechanism of lipiodol pneumonitis after TACE in HCC patients has not yet been studied in detail, and further studies are needed. We consider that conservative treatment is sufficient in terms of the clinical and radiological outcomes of lipiodol pneumonitis without respiratory failure, even if lipiodol accumulation is observed on CT after TACE. The mainstay of therapy is supportive treatment with supplemental oxygen and systemic antibiotics. Steroid therapy can be considered to reduce inflammation caused by lipiodol, but the evidence for this is still weak. We have learned that early detection and proper conservative management can lead to good recovery in these patients even if lipiodol pneumonitis occurs.
Lipiodol and/or adriamycin themselves, the large hypervascular HCC, the presence of AV shunting, embolization via inferior phrenic artery, and the amount of chemoembolic agents may have contributed as probable risk factors of lipiodol pneumonitis. The pre-TACE occlusion process of AV shunting or preferential RT may be a good alternative in preventing lipiodol pneumonitis. However, if AV shunting is identified but chemoembolization is considered essential, it may be possible to perform the procedure while monitoring the patient carefully. Physicians should be familiar with the clinical and radiographic presentations of lung toxicity associated with chemoembolic drugs and early detection and appropriate management of lipiodol pneumonitis.
REFERENCES
1. Dai H, Ding H, Liu F, Yao Z, Li L, Li C, et al. Complications of chemoembolization for hepatic neoplasms. Saudi Med J. 2007; 28:1208–1212.
2. Cho SH, Kim JH, Kim BS, Jang J. A case of acute lung injury complicated by transcatheter arterial chemoembolization for hepatocellular carcinoma. Tuberc Respir Dis. 1995; 42:781–786.
3. Zhao H, Wang HQ, Fan QQ, Chen XX, Lou JY. Rare pulmonary and cerebral complications after transarterial chemoembolization for hepatocellular carcinoma: a case report. World J Gastroenterol. 2008; 14:6425–6427.
4. Xu H, Yang R, Wang X, Zhu X, Chen H. Symptomatic pulmonary lipiodol embolism after transarterial chemoembolization for hepatic malignant tumor: clinical presentation and chest imaging findings. Chin Med J (Engl). 2014; 127:675–679.
5. Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y. al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006; 59:407–412.
6. Kim SI, Kim YR, Heo HM, Bae SE, Lee MW, Choi YJ, et al. A case of lipiodol pneumonitis after transarterial chemoembolization for hepatocellular carcinoma. Ewha Med J. 2009; 32:85–89.
7. Kim HW, Kim YH, Yoon HJ, Lee KH, Joo SM, Byun MK, et al. Lipiodol-induced pneumonitis following transarterial chemoembolization for ruptured hepatocellular carcinoma. Yeungnam Univ J Med. 2014; 31:117–121.
8. Okayasu I, Hatakeyama S, Yoshida T, Yoshimatsu S, Tsuruta K, Miyamoto H, et al. Selective and persistent deposition and gradual drainage of iodized oil, Lipiodol in the hepatocellular carcinoma after injection into the feeding hepatic artery. Am J Clin Pathol. 1988; 90:536–544.
9. Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993; 187:689–693.
10. Jouneau S, Vauléon E, Caulet-Maugendre S, Polard E, Volatron AC, Meunier C, et al. 131I-labeled lipiodol-induced interstitial pneumonia: a series of 15 cases. Chest. 2011; 139:1463–1469.
11. Lin MT, Kuo PH. Pulmonary lipiodol embolism after transcatheter arterial chemoembolization for hepatocellular carcinoma. JRSM Short Rep. 2010; 1:6.
12. Moon TY, Kim BH, Koo BS, Lee JW, Lee JW, Kim BS. Experimental study on the toxicity of lipiodol injected into proper hepatic artery of rabbits. J Korean Radiol Soc. 1990; 26:449–461.
13. Youn IY, Chong SM, Kwak BK, Shim HJ, Seo GY, Seo JS, et al. Pulmonary lipiodol accumulation after transarterial chemoembolization: CT findings and its radiologic outcomes. J Korean Soc Radiol. 2011; 65:577–583.
14. Wu GC, Perng WC, Chen CW, Chian CF, Peng CK, Su WL. Acute respiratory distress syndrome after transcatheter arterial chemoembolization of hepatocellular carcinomas. Am J Med Sci. 2009; 338:357–360.
15. Morse SS, Sniderman KW, Galloway S, Rapoport S, Ross GR, Glickman MG. Hepatoma, arterioportal shunting, and hyperkinetic portal hypertension: therapeutic embolization. Radiology. 1985; 155:77–82.
16. Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997; 52:36–40.
17. Nishiofuku H, Tanaka T, Sakaguchi H, Yamamoto K, Inoue M, Sueyoshi S, et al. Hepatic arterial infusion chemotherapy combined with venous embolization in a patient with hepatic metastases with an arteriovenous shunt. Cardiovasc Intervent Radiol. 2009; 32:796–800.
18. Khan I, Vasudevan V, Nallagatla S, Arjomand F, Ali R. Acute lung injury following transcatheter hepatic arterial chemoembolization of doxorubicin-loaded LC beads in a patient with hepatocellular carcinoma. Lung India. 2012; 29:169–172.
19. Lee JH, Won JH, Park SI, Won JY, Lee do Y, Kang BC. Transcatheter arterial chemoembolization of hepatocellular carcinoma with hepatic arteriovenous shunt after temporary balloon occlusion of hepatic Vein. J Vasc Interv Radiol. 2007; 18:377–382.
20. Koc O, Cil BE, Peynircioglu B, Emlik D, Ozbek O. Complementary use of NBCA with the Amplatzer vascular plug for embolization of a high-flow traumatic hepatic arteriovenous fistula. Cardiovasc Intervent Radiol. 2009; 32:1105–1107.
Figure 1.
Magnetic resonance imaging (MRI) findings. MRI showed a well-defined, round lesion with low signal intensity on unenhanced T1-weighted imaging (A). This lesion showed enhancement in the arterial (B) phase and washout on portal (C) and hepatobiliary (D) phases.
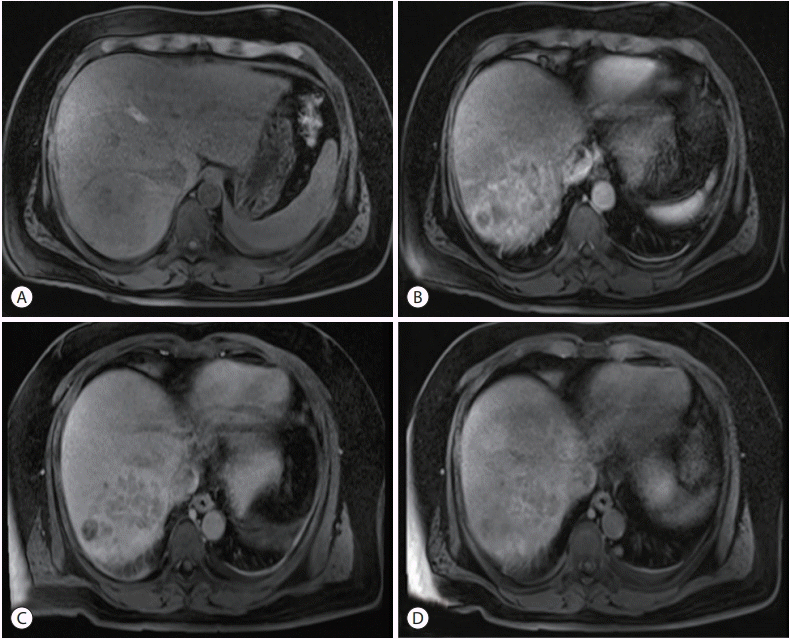
Figure 2.
Angiography and computed tomography findings. The angiogram shows the findings of hepatocellular carcinoma supplied from the right hepatic artery and its segment 4 branch. It also shows arteriovenous shunting between the hepatic artery and hepatic vein. Infusion of an adriamycin and lipiodol mixture and subsequent embolization were performed (A). Abdominal computed tomography after transcatheter arterial chemoembolization showing a lipiodol-laden mass lesion in the right hemiliver (B).
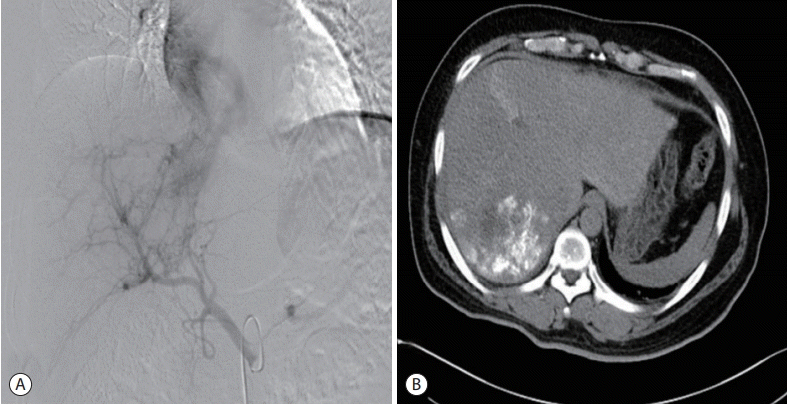
Figure 3.
Chest computed tomography (CT) findings. On the first day after transcatheter arterial chemoembolization, a non-enhanced chest CT shows consolidation with scattered lipiodol accumulation (arrows) in both lungs (A, B).
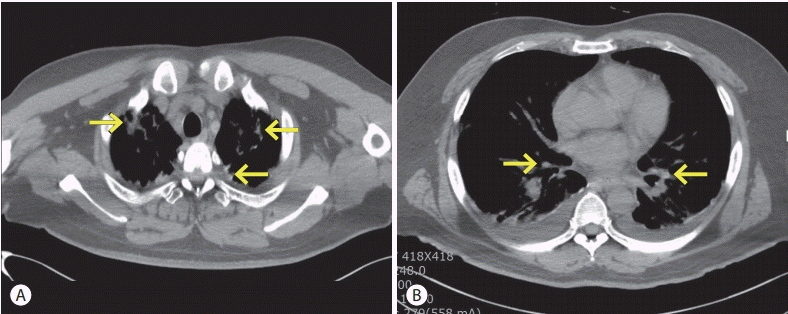
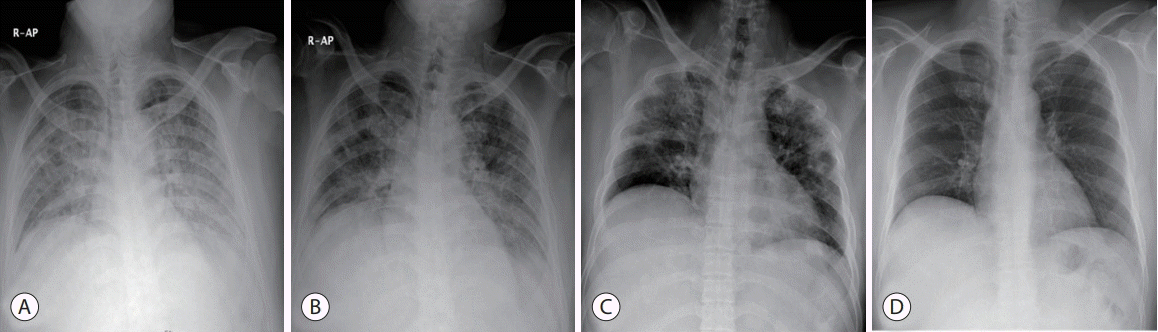
Figure 4.
Follow-up chest X-ray findings. Multiple patches and diffuse ground-glass infiltrations were found in the bilateral lung on the first day (A) after transcatheter arterial chemoembolization (TACE). A follow-up chest X-ray showed progressive improvement of the infiltration of both lung fields on day 3 (B) and day 7 (C) after TACE. The last chest X-ray shows nearly complete resolution of lung infiltrations on day 14 (D). R-AP, right-anterior posterior.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download