Abstract
Backgrounds/Aims
Hepatic arterial infusion chemotherapy (HAIC) has been reported as an effective treatment for advanced hepatocellular carcinoma. The aim of this study is to compare the effect and safety between a high-dose regimen (750 mg/m2 5-fluorouracil [FU] and 25 mg/m2 cisplatin on day 1-4) and a low-dose regimen (500 mg/m2 5-FU on day 1-3 with 60 mg/m2 cisplatin on day 2).
Methods
A total of 48 patients undergoing HAIC were retrospectively analyzed. Thirty-two patients were treated with the high-dose and 16 patients with the low-dose regimen.
Results
Complete response (CR), partial response (PR), stable disease (SD), and progressive disease were noted in one (3.1%), 15 (46.9%), three (9.4%), and 13 patients (40.6%) in the high-dose group, and 0 (0%), one (6.3%), eight (50%), and seven patients (43.8%) in the low-dose group (p=0.002). The disease control rate (CR, PR, and SD) did not differ between groups (59.4% vs. 56.3%, p=1.000), but the objective response rate (CR and PR) was significantly higher in the high-dose group (50.0% vs. 6.3%, p=0.003). The median progression free survival did not differ between groups (4.0 vs. 6.0, p=0.734), but overall survival was significantly longer in the high-dose group (not reached vs. 16.0, p=0.028). Fourteen (43.8%) patients in the high-dose group and two patients (12.5%) in the low-dose group experienced grade 3-4 toxicities (p=0.050).
Approximately 10-40% of hepatocellular carcinoma (HCC) is diagnosed at an advanced stage with portal vein invasion [1,2]. Although sorafenib is a mainly used for treatment of advanced HCC, transarterial chemoembolization (TACE), radiotherapy and hepatic arterial infusion chemotherapy (HAIC) can be other options. In a study of advanced HCC cases in the Asian-Pacific area, patients treated with sorafenib survived only 2 to 3 months longer than the control group [3]. According to many studies, combined treatments resulted in better outcomes compared to sorafenib alone [4].
In HAIC, chemo-agents are infused directly into the hepatic artery through an implanted catheter, providing higher local concentrations and lower systemic side effects [5,6]. Although it has clinical efficacy for advanced HCC with portal vein thrombosis including infiltrating types, HAIC has limitations for standardized therapy due to lack of unified protocols, diverse indications, differences in response rate, deterioration of liver and bone marrow function, catheter-related problems, and other issues [7]. In spite of variable response rates from 7 to 81%, some studies have reported a dramatic response to HAIC in some patients with advanced HCC. A multicenter study in South Korea showed that patients treated with HAIC had better outcomes than those treated with sorafenib for advanced HCC with portal vein invasion [8-10].
There are several kinds of chemo-agents for HAIC, of which a combination of 5-fluorouracil (5-FU) and cisplatin is the most commonly used [11]. The efficacy of this regimen varies from 5.0-19.5 months of median overall survival (OS) and 3.8-38.5% of response rate. These unstable outcomes are due to stage of cancer, existence of portal vein thrombosis, baseline liver function, and dose of chemo-agents etc [7,11-13]. High-dose chemotherapy usually has better efficacy than low-dose chemotherapy, but it carries a higher risk of deteriorating liver function or of having side effects [7]. There is a lack of studies on the optimal dose of chemo-agents in HAIC. Therefore, we aimed to compare the efficacy and safety of high-dose 5-FU/cisplatin to that of low-dose 5-FU/cisplatin.
This study was conducted as a retrospective cohort study. We reviewed the medical records of patients who were treated with HAIC between September 2010 to December 2016. Inclusion criteria were as follows: 1) HCC with main portal vein invasion, 2) inadequate for TACE (large arterioportal shunt or infiltrating HCC), 3) refractory to TACE, 4) preserved baseline liver function below Child-Push score 7, 5) adequate marrow function with absolute neutrophil count > 1,500/mm3, leukocyte count >3,000/mm3 and platelet count >70,000/mm3, 6) serum creatinine <1.5 mg/dL, 7) Eastern Cooperative Oncology Group (ECOG) performance score 0-1, and 8) age between 19 and 71. Exclusion criteria were as follows:7 1) baseline liver function above Child-Pugh score 7, 2) huge HCC covering over 50% of the surface of the liver, 3) significant decompensation such as variceal bleeding, uncontrolled ascites, or hepatic encephalopathy within the last 6 months, 4) history of an another systemic chemotherapy, and 5) other uncontrolled malignancies or comorbidities. This study is approved by clinical research ethics committee of Chonnam National University Hwasun Hospital (CNUHH-2016-011) and it is in observance of the Helsinki declaration.
Based on studies previously reported in South Korea, patients were divided into the high- and low-dose groups [8,14,15]. The high-dose regimen consisted of infusing 750 mg/m2 5-FU and 25 mg/m2 cisplatin for 4 days daily and the lowdose regimen was an infusion of 500 mg/m2 5-FU for 3 days daily with 60 mg/m2 cisplatin on day 2.
Baseline imaging was obtained using dynamic contrast-enhanced computed tomography or magnetic resonance imaging before the treatment. Follow-up images were checked every 2 or 3 cycles, and tumor response was assessed. Each cycle of HAIC was performed every 3 to 4 weeks, and blood test and physical examination were checked before each cycle. If the blood test and physical examination showed that patient was not fully recovered from toxicities, dose of chemo-agents was reduced by 20 to 25 percents or the next cycle of HAIC was delayed.
OS was defined as the primary outcome and progressionfree survival (PFS), response rate, and adverse events were used as secondary outcomes. The OS was defined as the duration from the start of HAIC to death or the last follow-up. The PFS was defined as the duration from the start of HAIC to the date of disease progression or death. Treatment response was assessed as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to modified Response Evaluation Criteria in Solid Tumors (mRECIST), and the best response during the treatment was used for analysis. Objective response rate was defined as the proportion of patients with CR or PR, and disease control rate was defined as the proportion of patients with CR, PR, or SD. Treatment-related adverse events were assessed based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Results were reported as mean (±standard deviation), number (%), or median (95% CI) and comparisons were performed using Student’s t-tests, Chi-square tests or Fisher’s exact tests. Survival outcomes were estimated using the Kaplan-Meier method and compared by log-rank tests. Univariate analyses and multivariate analyses by Cox regression model were used to analyze factors that affect survival. P-values less than 0.05 were regarded as statistically significant. All statistical analyses were performed by Statistical Package for the Social Sciences (IBM SPSS ver. 18.0; IBM Corp., Armonk, NY, USA).
A total of 48 patients were analyzed, which consist of 32 patients in the high-dose group and 16 patients in the low-dose group. The two groups did not differ significantly in age, gender, ECOG performance score, etiology of liver disease, Child-Pugh score, Model for End-Stage Liver Disease score, presence of ascites, or previous use of antiviral agent. The number of patients with portal vein invasion was 19 (59.4%) in the high-dose group and 12 (75.0%) in the low-dose group. And the number of patients with extrahepatic metastasis was one (3.1%) in the high-dose group and three (18.8%) in the low-dose group. There were five patients (15.6%) with Barcelona Clinic Liver Cancer (BCLC) stage B and 27 (84.4%) with BCLC stage C in the high-dose group, and all of the low-dose group had BCLC stage C. For modified Union of International Cancer Control stage, there were five patients (15.6%) with stage III, 26 (81.3%) of stage IVa and one (3.1%) with stage IVb in the high-dose group, and there were 13 patients (81.3%) with stage IVa and three (18.8%) with stage IVb in the low-dose group. Twenty patients (62.5%) in the high-dose group and seven (43.8%) in the low-dose group were previously treated with other therapies before HAIC. The most common previous therapy was TACE, 19 patients (59.4%) in the high-dose group and five patients (31.3%) in the low-dose group. Serum alpha-fetoprotein (AFP) and protein-induced by vitamin K absence or antagonist –II (PIVKA-II) values were not significantly different between the two groups. The mean number of HAIC cycles was 5.2 in the high-dose group and 6.7 in the low-dose group, and the mean duration of HAIC treatment was 156.3 days for high-dose group patients and 189.3 days for lowdose group patients. Seventeen patients (53.1%) in the high-dose group and seven (43.8%) in the low-dose group underwent rescue therapy after disease progression (Table 1).
According to mRECIST, CR, PR, SD, and PD were noted in one (3.1%), 15 (46.9%), three (9.4%), and 13 patients (40.6%) in the high-dose group, and 0 (0%), one (6.3%), eight (50.0%), and seven patients (43.8%) in the low-dose group, respectively (P=0.002). The disease control rate (CR, PR, and SD) did not differ between groups (59.4% vs. 56.3%, P=1.000), but the objective response rate (CR and PR) was significantly higher in the high-dose group (50.0% vs. 6.3%, P=0.003), (Table 2).
The median follow-up time was 12.3 months (range, 1 to 39 months) in the high-dose group and 11.8 months (range, 1 to 26 months) in the low-dose group, respectively. Median PFS was 6.9 months (range, 0 to 26 months) in the high-dose group and 6.4 months (range, 0-16 months) in the low-dose group (P=0.734, Fig. 1A).
During the follow-up period, 28.1% (9/32) in the high-dose group patients and 87.5% (14/16) of low-dose group patients died. Median OS was not reached in the high-dose group and 16.0 months (range, 1-26 months) in the lowdose group (P=0.028, Fig. 1B). Furthermore, in 43 patients (27 in the high dose group, 16 in the low-dose group) with BCLC stage C, no significant difference in PFS and OS was observed between groups (PFS, 3.0 months vs. 6.0 months, P=0.612; OS, not reached vs. 16.0 months, P=0.083). The cox regression model analysis showed that age (HR, 1.076; 95% CI, 1.017-1.138; P=0.010), baseline AFP value over 200 ng/mL (HR, 6.423; 95% CI, 1.570-26.271; P=0.010), and decrease of PIVKA-II value less than 25% (HR, 7.184; 95% CI, 1.191-43.335; P=0.032) were significant prognostic factors for OS (Table 3).
The frequency of total adverse events was similar, 90.6% (29/32) in the high-dose group and 87.5% (14/16) in the lowdose group. However, 43.8% (14/32) of the high-dose group patients and 12.5% (2/16) of the low-dose group patients experienced severe adverse events (grade 3-4) (P=0.050). The most common adverse event over grade 2 in the high-dose group was neutropenia (25.0%) (Table 4).
The prognosis of advanced HCC was poor with median OS of 2 to 4 months despite aggressive treatment [1]. According to the newly revised guideline in South Korea, sorafenib or lenvatinib is recommended as first-line treatment for advanced HCC patients with vascular invasion or extrahepatic spread, and preserved liver function. And HAIC is considered for targeted agent-refractory or intolerant HCCs with portal vein invasion. HAIC is not globally recommended therapy in guidelines because of lack of well-designed randomized controlled trials, various indications for each institution, absence of standardized protocols.
Many studies showed various results on response to HAIC. The objective response rate of the 5-FU/cisplatin regimen that most widely used in South Korea was reported as 22 to 32%, and CR rate was also reported in 2 to 6% [8,11,14,15]. In a prospective multicenter study for advanced HCC with portal vein thromobosis in South Korea, HAIC was superior to sorafenib in both treatment response and survival (HAIC; CR 2%, PR 22%, SD 66%, PD 10%, OS 7.1 months vs. Sorafenib; CR 0%, PR 13%, SD 32%, PD 55%, OS 5.5 months) [8].
Higher dose of chemotherapy is expected to have better response but greater toxicities by deteriorating liver function [7]. An adequate dose of chemotherapy to optimize response and minimize toxicities is not yet established, but greater efficacy is expected with higher doses for patients with Child-Pugh A liver function and preserved bone marrow function.
In this study, patients were divided into the high-dose group (total dose for one cycle; 3,000 mg/m2 5-FU and 100 mg/m2 cisplatin) and the low-dose group (1,500 mg/m2 5-FU and 60 mg/m2 cisplatin) based on previous reports in South Korea. The high-dose group in this study was infused separated dose daily for a few days rather than infusing at once, in order to increase whole dose of chemo-agents and prolong duration of exposure to chemo-agents. In the high-dose group had a better objective response rate (high-dose group, 50.0% vs. low-dose group, 6.3%), and a higher incidence of severe adverse events over grade 2 (high-dose group, 43.8% vs. lowdose group, 12.5%). But there were no clinically uncontrolled adverse events in both two groups. Compared to another study using high-dose of cisplatin and 5-FU, this study showed a better objective response rate and a similar incidence of hematologic adverse events [14]. These results may be attributed to baseline characteristics of the patients, exclusion of large HCC covering over 50% of the liver surface and inclusion of patients with TACE refractoriness in BCLC stage B.
The duration of HAIC treatment is not established. HAIC does not always guarantee good response and further study should be done to establish how many cycles are adequate. Repeated and long-term exposure to the same chemo-agents may make intolerance of HCC, and cause liver function deterioration, bone marrow suppression, and malnutrition due to toxicities [16]. So we thought that more than six cycles of HAIC should be avoided. In this study, one patient who underwent 18 cycles of HAIC became weak and cachexic, and finally died of pneumonia. And early prediction of tumor response is possible because significant decrease in tumor markers and tumor sizes after 1-2 cycles is observed in most of patients with good response to HAIC [17,18]. So we finished HAIC early in patients who showed PD after 2 cycles, and continued HAIC up to 6 cycles for those who showed SD and PR after 2 cycles. And sequential treatments such as resection, TACE, target therapy, and radiotherapy were carried out according to the status of tumor and patients. Therefore, it is necessary to evaluate the role of HAIC as a bridging therapy to decrease size of tumor and to improve efficacy of sequential rescue therapy such as targeted agents rather than maintenance therapy [19,20].
This study has some limitations. First, this study is a single center, retrospective study with many biases. Second, there were heterogenous stages in the two groups with more intermediate stage patients in the high-dose group, and this factor affected treatment response and survival.
In conclusion, HAIC with high-dose 5-FU/Cisplatin may achieve better tumor response and may improve survival compared to a low-dose regimen for advanced HCC with preserved liver and bone marrow function. However, high-dose regimens should be administered cautiously because of higher incidence of adverse events. More studies are needed to determine the adequate cycles of HAIC, and this should establish a role HAIC as a bridging therapy combined with other options such as target therapy.
REFERENCES
1. Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006; 12:7561–7567.
2. Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso MC, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999; 29:62–67.
3. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
4. Jun CH, Yoon JH, Cho E, Shin SS, Cho SB, Kim HJ, et al. Barcelona clinic liver cancer-stage C hepatocellular carcinoma: a novel approach to subclassification and treatment. Medicine (Baltimore). 2017; 96:e6745.
5. Bartkowski R, Berger MR, Aguiar JL, Henne TH, Dörsam J, Geelhaar GH, et al. Experiments on the efficacy and toxicity of locoregional chemotherapy of liver tumors with 5-fluoro-2’-deoxyuridine (FUDR) and 5-fluorouracil (5-FU) in an animal model. J Cancer Res Clin Oncol. 1986; 111:42–46.
6. Kuan HY, Smith DE, Ensmiger WD, Knol JA, DeRemer SJ, Yang Z, et al. Regional pharmacokinetics of 5-bromo-2’-deoxyuridine and 5-fluorouracil in dogs: hepatic arterial versus portal venous infusions. Cancer Res. 1996; 56:4724–4727.
7. Woo HY, Bae SH, Park JY, Han KH, Chun HJ, Choi BG, et al. A randomized comparative study of high-dose and low-dose hepatic arterial infusion chemotherapy for intractable, advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2010; 65:373–382.
8. Song DS, Song MJ, Bae SH, Chung WJ, Jang JY, Kim YS, et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J Gastroenterol. 2015; 50:445–454.
9. Kondo M, Morimoto M, Ishii T, Nozaki A, Fukuda H, Numata K, et al. Hepatic arterial infusion chemotherapy with cisplatin and sorafenib in hepatocellular carcinoma patients unresponsive to transarterial chemoembolization: a propensity score-based weighting. J Dig Dis. 2015; 16:143–151.
10. Baek YH, Kim KT, Lee SW, Jeong JS, Park BH, Nam KJ, et al. Efficacy of hepatic arterial infusion chemotherapy in advanced hepatocellular carcinoma. World J Gastroenterol. 2012; 18:3426–3434.
11. Song DS, Bae SH, Song MJ, Lee SW, Kim HY, Lee YJ, et al. Hepatic arterial infusion chemotherapy in hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2013; 19:4679–4688.
12. Lim TY, Cheong JY, Cho SW, Sim SJ, Kim JS, Choi SJ, et al. Effect of low dose 5-fluorouracil and cisplatin intra-arterial infusion chemotherapy in advanced hepatocellular carcinoma with decompensated cirrhosis. Korean J Hepatol. 2006; 12:65–73.
13. Yamashita T. Current status of hepatocellular carcinoma treatment in Japan: hepatic arterial infusion chemotherapy. Clin Drug Investig. 2012; 32 Suppl 2:15–23.
14. Oh MJ, Lee HJ, Lee SH. Efficacy and safety of hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma as firstline therapy. Clin Mol Hepatol. 2013; 19:288–299.
15. Song MJ, Bae SH, Chun HJ, Choi JY, Yoon SK, Park JY, et al. A randomized study of cisplatin and 5-FU hepatic arterial infusion chemotherapy with or without adriamycin for advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2015; 75:739–746.
16. Thomas MB. Systemic therapy for hepatocellular carcinoma. Cancer J. 2008; 14:123–127.
17. Miyaki D, Kawaoka T, Aikata H, Kan H, Fujino H, Fukuhara T, et al. Evaluation of early response to hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma using the combination of response evaluation criteria in solid tumors and tumor markers. J Gastroenterol Hepatol. 2015; 30:726–732.
18. Lin CC, Hung CF, Chen WT, Lin SM. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein thrombosis: impact of early response to 4 weeks of treatment. Liver Cancer. 2015; 4:228–240.
19. Kudo M, Ueshima K, Yokosuka O, Ogasawara S, Obi S, Izumi N, et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): a randomised, open label, phase 3 trial. Lancet Gastroenterol Hepatol. 2018; 3:424–432.
20. Kodama K, Kawaoka T, Aikata H, Uchikawa S, Nishida Y, Inagaki Y, et al. Comparison of outcome of hepatic arterial infusion chemotherapy combined with radiotherapy and sorafenib for advanced hepatocellular carcinoma patients with major portal vein tumor thrombosis. Oncology. 2018; 94:215–222.
Figure 1.
Comparison of (A) progression-free survival and (B) overall survival between high-dose and low-dose regimen.
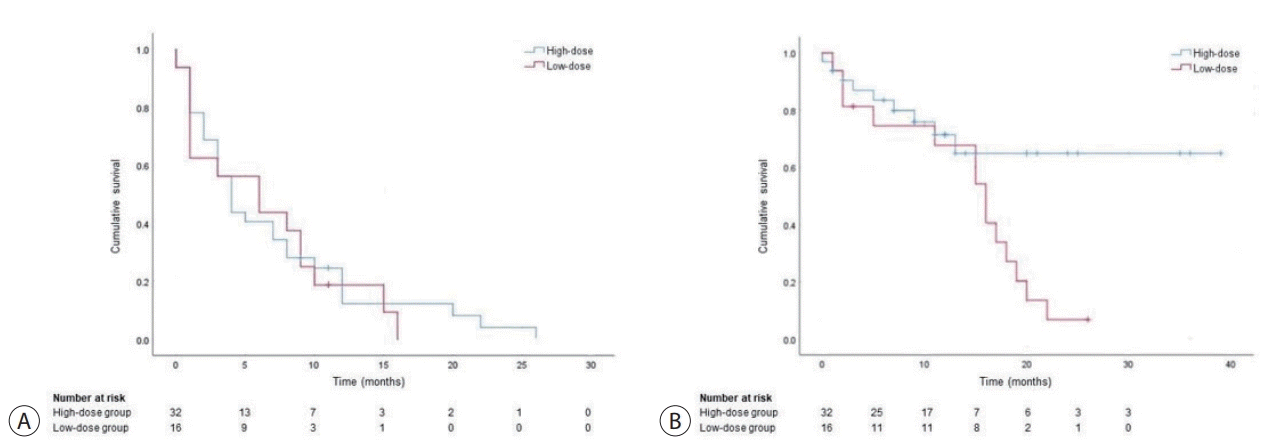
Table 1.
Baseline demographic and clinical characteristics
| High-dose group (n=32) | Low-dose group (n=16) | P-value* | |
|---|---|---|---|
| Age (years) | 57.2 ± 8.7 | 56.9 ± 12.5 | 0.741 |
| Sex (male) | 26 (81.3) | 16 (100.0) | 0.159 |
| ECOG performance status | 0.457 | ||
| 0 | 27 (84.4) | 12 (75.0) | |
| 1 | 5 (15.6) | 4 (25.0) | |
| Etiology | 0.414 | ||
| HBV | 26 (81.3) | 11 (69.8) | |
| HCV | 1 (3.1) | 1 (6.3) | |
| Alcohol | 5 (15.6) | 4 (25.0) | |
| Child Pugh Class | 0.468 | ||
| A | 26 (81.3) | 11 (68.8) | |
| B | 6 (18.8) | 5 (31.3) | |
| MELD Score | 8.2 ± 1.6 | 9.1 ± 2.4 | 0.318 |
| Ascites | 16 (50.0) | 4 (25.0) | 0.127 |
| Antiviral therapy | 17 (65.4) | 9 (81.8) | 0.445 |
| Portal vein invasion | 19 (59.4) | 12 (75.0) | 0.350 |
| Extrahepatic metastasis | 1 (3.1) | 3 (18.8) | 0.101 |
| BCLC stage | 0.154 | ||
| Stage B | 5 (15.6) | 0 (0.0) | |
| Stage C | 27 (84.4) | 16 (100.0) | |
| Modified UICC stage | 0.057 | ||
| Stage III | 5 (15.6) | 0 (0.0) | |
| Stage IVA | 26 (81.3) | 13 (81.3) | |
| Stage IVB | 1 (3.1) | 3 (18.8) | |
| Previous treatment | 20 (62.5) | 7 (43.8) | 0.355 |
| Surgery | 9 (28.1) | 0 (0.0) | |
| Radiofrequency ablation | 8 (25.0) | 1 (6.3) | |
| TACE | 19 (59.4) | 5 (31.3) | |
| Radiotherapy | 1 (3.1) | 2 (12.5) | |
| Sorafenib | 5 (15.6) | 2 (12.5) | |
| AFP level (ng/mL) | 7,274.1 ± 11,017.2 | 8,922.2 ± 11,762.3 | 0.944 |
| PIVKA-II level (mAU/mL) | 6,750.8 ± 10,943.0 | 2,649.8 ± 3,543.3 | 0.412 |
| Number of cycle | 5.2 ± 3.5 | 6.7 ± 5.2 | 0.590 |
| Duration of treatment (days) | 156.3 ± 154.9 | 189.3 ± 174.1 | 0.766 |
| Rescue treatment | 17 (53.1) | 7 (43.8) | 0.760 |
Values are presented as mean ± standard deviation or number (%).
ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; BCLC, Barcelona Clinic Liver Cancer; UICC, Union of International Cancer Control; TACE, transcatheter arterial chemoembolization; AFP, alpha-fetoprotein; PIVKA-II, protein-induced by vitamin K absence or antagonist-II.
Table 2.
Treatment response by modified RECIST criteria
| High-dose group (n=32) | Low-dose group (n=16) | P-value* | |
|---|---|---|---|
| Best response | 0.002 | ||
| Complete response (CR) | 1 (3.1) | 0 (0.0) | |
| Partial response (PR) | 15 (46.9) | 1 (6.3) | |
| Stable disease (SD) | 3 (9.4) | 8 (50.0) | |
| Progressive disease (PD) | 13 (40.6) | 7 (43.8) | |
| Objective response rate (CR+PR) | 16 (50.0) | 1 (6.3) | 0.003 |
| Disease control rate (CR+PR+SD) | 19 (59.4) | 9 (56.3) | 1.000 |
Table 3.
Factors associated with overall survival according to univariate and multivariate analyses
Table 4.
Treatment-related adverse events




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download