Abstract
Background
The STOP-Bang questionnaire is a simple screening tool with high sensitivity for the detection of severe obstructive sleep apnea. Predicting airway obstruction would allow the safe management of sedative patients to prevent intraoperative hypoxia. This study was designed to check the correlation between the STOP-Bang score and oxygen saturation (SpO2) during sedation and confirm the availability of the STOP-Bang questionnaire as a preoperative exam for predicting the incidence of hypoxia in sedative patient management.
Methods
This study included 56 patients who received spinal anesthesia. The pre-anesthesia evaluation was conducted using the STOP-Bang questionnaire. The patients were under spinal anesthesia with an average block level of T10. Dexmedetomidine was infused with a loading dose of 1 μg/kg over 10 min and a maintenance dose of 0.5 μg/kg/h until the end of the procedure. The SpO2 of the patients was recorded every 5 min.
Results
The STOP-Bang score was negatively correlated with the lowest SpO2 (coefficient = –0.774, 95% confidence interval [CI]: –0.855 to –0.649, standard error [SE] = 0.054, P < 0.001). The item of “observed apnea” was the most correlated one with hypoxic events (odds ratio = 6.00, 95% CI: 1.086 to 33.145).
For regional anesthesia, sedation is widely used for patient comfort and is required in several places in the hospital as well as in the operating room [1]. Dexmedetomidine has a lesser effect on the respiratory system than other sedative drugs such as propofol or benzodiazepines, especially for short-term sedation such as for drug-induced sleep endoscopy [2,3]. However, studies have shown that central sleep apnea does not occur, but obstructive sleep apnea (OSA) may worsen [4]. Of the complications related to sedation, airway obstruction can cause hypoventilation and hypoxia [5]. If hypoxia persists during surgery, stable sedation will be impossible, and the patient may wake up during the procedure, which may cause an interruption or necessitate unintended discontinuation [6].
In this regard, OSA is the most common sleep disorder, mainly due to upper respiratory tract obstruction with a prevalence of 10% in men and 3% in women aged between 30 and 49 years, and approximately 17% of men and 9% of women aged between 50 and 70 years. The incidence of hypoxemia in patients at risk for OSA is high [7]. Likewise, the incidence of difficult mask ventilation or difficult intubation is high [8]. Polysomnography, the standard method for diagnosing OSA, is not time- and cost-effective to apply to all patients for preoperative risk evaluation of OSA.
The STOP-Bang questionnaire uses only eight items to calculate the risk of OSA. The eight items include the patient's body mass index (BMI, gender , age, neck circumference, hypertension under treatment, daily drowsiness, snoring, and apnea during sleep [9,10]. It does not require such a long time, and patients feel more comfortable with it. Although it seems simple compared with other screening tools, its sensitivity is sufficiently high to be reliable in clinical fields [11]. Therefore, the STOP-Bang questionnaire, which has the advantages of simple questions and high sensitivity over other OSA screening tools [12,13], was used for this study to identify patients at high-risk of hypoxemia before sedation.
The authors hypothesized that the STOP-Bang score would be correlated with the incidence of hypoxic events during sedation in patients undergoing spinal anesthesia. The STOP-Bang score can be used to predict the degree of desaturation in sedated patients.
The primary outcome of the study was to check the correlation between the STOP-Bang score and oxygen saturation during dexmedetomidine sedation. Additional attempts were made to determine the cut-off value of the STOP-Bang score that can predict hypoxia during sedation and the items with the highest diagnostic values.
This study was approved by the Institutional Review Board (no. KUGH 2019-11-032). Preoperative screening of orthopedic and urological patients who underwent sedation with dexmedetomidine after spinal anesthesia was performed. The exclusion criteria were as follows: American Society of Anesthesiologists physical status class of ≥ 3, lateral decubitus or prone positioning during surgery, problems of the upper respiratory tract, history of severe asthma or chronic obstructive lung disease, refusal of intraoperative sedation, or sensory block higher than T5. Patients with American Society of Anesthesiologists physical status class 3 due to a high BMI value of > 40 kg/m2 without any other underlying diseases were included for consideration of the purpose of the study. Written informed consent was obtained from all the patients.
The STOP-Bang score of the patient was confirmed through pre-anesthesia evaluation. The STOP-Bang questionnaire consists of four questions and four clinical characteristics. The STOP-Bang score ranged from 0 to 8 points based on eight items (Supplementary Figs. 1, 2; S = Snoring; T = Tiredness; O = Observed apnea; P = Being treated for high blood pressure; B = BMI over 35 kg/m2; A = age over 50 years; N = neck circumference over 43 cm in males and 41 cm in females; G = gender of males). Patients were classified based on the STOP-Bang questionnaire scores into three groups: the high-risk, intermediate-risk, and low-risk of OSA groups (low-risk group, 0–2 points; intermediate-risk group, 3–4 points; high-risk group, 5–8 points or 2–4 points of STOP questions with at least one of B, N, and G). The official STOP-Bang questionnaire format was used in the design of the study; however, to be considerate of patients, an official Korean translation version was used when surveying the patients. Both forms were provided at www.STOPBANG.ca by Chung et al. [13] .
The patients were placed in the right or left lateral position for spinal anesthesia while monitoring their noninvasive blood pressure, electrocardiogram, and pulse saturation. After disinfection of the skin with povidone-iodine solution, a 25-gauge spinal needle was used in a median approach between the 3rd and 4th or the 4th and 5th lumbar spinous process while checking the free flow of cerebrospinal fluid, and a heavy bupivacaine 10–12 mg was injected. Immediately after the removal of the spinal needle, the patient was placed in the supine position and waited for approximately 10 minutes to check the sensory block height using the pinprick test. Dexmedetomidine was used for patient sedation during the surgery with a loading dose of 1 μg/kg for 10 min followed by a maintenance dose at a rate not exceeding 0.5 μg/kg/h until the end of the surgery. During dexmedetomidine sedation, vital signs, including SpO2, were measured and recorded at 5-min intervals. End-tidal capnography was used to confirm the apnea during sedation. The oxygen mask was applied immediately to the patient when the SpO2 during sedation decreased to 92% and lasted more than 30 s or rapidly decreased to 90% or less. In such cases, maintenance dose adjustment and adequate stimulation to wake up the patient were provided, if necessary. Rescue steps, such as jaw thrust or manual ventilation, were planned as needed.
According to a previous study by Oshita et al. [14] and the pilot data from our center, we assumed that the correlation between the STOP-Bang score and the lowest SpO2 was 0.4 in calculating sample size. Assuming an attrition rate of 10%, a sample size of 51 patients were determined to be an adequate sample size to aim for 80% power and 5% type-1 error.
All statistical analyses were performed using IBM SPSS version 20.0 (IBM Co., USA). The correlation between the lowest SpO2 and the STOP-Bang scores, age, and BMI were analyzed using simple linear regression and multiple linear regression analysis. The receiver operating characteristic (ROC) curve and the area under the curve (AUC) values were calculated to determine the diagnostic usefulness of hypoxic events and the cut-off value of the STOP-Bang score, age, and BMI. The Mann–Whitney U test and the chi-square test were used to compare the age and BMI of the hypoxic and non-hypoxic groups. The correlations between the individual items of the STOP-Bang questionnaire and the hypoxic events were also analyzed with odds ratios and 95% confidence intervals (CI). Two-sided P values of < 0.05 represented statistical significance.
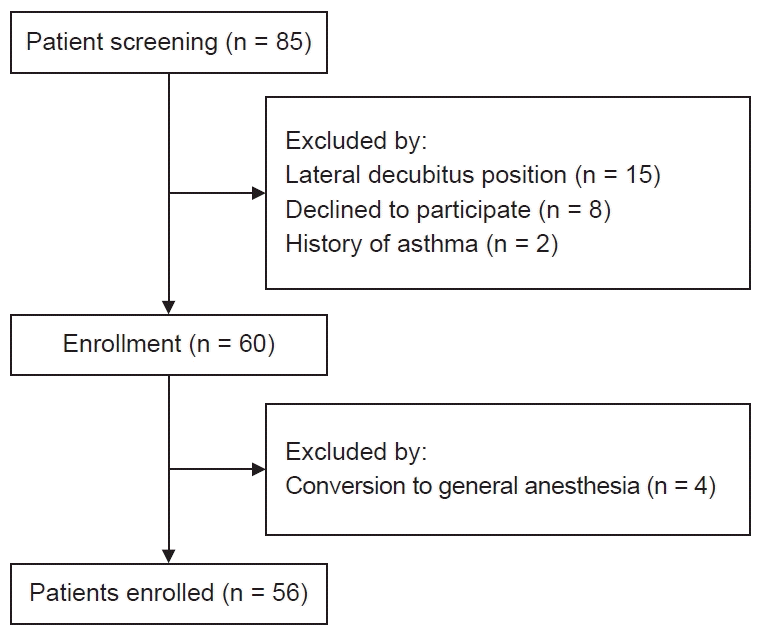
From February 2020 to May 2020, 60 patients were enrolled in the present study. Four patients dropped out due to conversion from spinal anesthesia to general anesthesia (Fig. 1). The patient characteristics, level of spinal block, sedation time, and the STOP-Bang questionnaire composition ratio are summarized in Table 1.
A negative correlation (coefficient = –0.774, 95% CI: –0.855 to –0.649, standard error [SE] = 0.054, P < 0.001) between the STOP-Bang score and the lowest SpO2 value was observed (Table 2). In addition to the STOP-Bang score, age and BMI, which are expected to be associated with hypoxia during sedation, were also evaluated using Spearman correlation analysis. Compared with the STOP-Bang score, BMI was weakly correlated with the lowest SpO2 (coefficient = –0.288, 95% CI: –0.518 to –0.013, SE = 0.142, P = 0.031) and age had no correlation with the lowest SpO2 (coefficients = –0.201, 95% CI: –0.418 to 0.032, SE = 0.114, P = 0.137). Scatter plots of the lowest oxygen saturation during sedation and the STOP-Bang score, age, and BMI are shown in Fig. 2.
The ROC analysis of the STOP-Bang score, age, and BMI was performed to determine their usefulness in predicting hypoxic events during sedation. Unlike age and BMI, only the STOP-Bang score was statistically significant (AUC = 0.943, 95% CI: 0.884 to 1.000, P < 0.001) as a diagnostic tool for hypoxia. The cut-off value of the STOP-Bang score was 4.
Among 8 items of the STOP-Bang questionnaire, 6 items of STOP were analyzed using odds ratio, and the item of “observed apnea” turned out to be the most correlated one with hypoxic events (Table 3). None of the patients had a BMI of > 35 kg/m2. Only one patient was included in the neck circumference section. Therefore, a statistical analysis of both items was not possible.
Oxygen masks were immediately applied to eight patients due to intraoperative SpO2 of < 92% lasting for more than 30 s or a sudden decrease in saturation to less than 90% (Table 4). In addition, three patients showed mild bradycardia and were administered glycopyrrolate. After glycopyrrolate injection and a reduction in the dexmedetomidine maintenance dose, the heart rate recovered.
Respiratory depression is a common problem associated with sedation. Dexmedetomidine, a selective α2 adrenergic receptor agonist, has sedative and analgesic properties. Its sedation is similar to that of natural sleep; therefore, it is known to cause less respiratory depression [15]. However, the advantage of being similar to natural sleep implies that the incidence rate of OSA is high, as reported by Shin et al. [16]. OSA has a causal relationship with hypoxia during sedation, and severe hypoxia may be associated with increased mortality and morbidity [6]. Predicting those who are more likely to experience hypoxia will help us prepare for hypoxia during sedation.
The Berlin questionnaire, the STOP-Bang questionnaire, and the Epworth sleepiness scale are widely used as OSA screening tools. Among them, the STOP-Bang questionnaire is relatively accurate with high sensitivity and can be used for the early diagnosis of OSA, even in an environment where polysomnography is difficult to perform [17]. In addition, the STOP-Bang questionnaire uses only eight items to calculate the risk of OSA. The eight items include BMI, gender, age, neck circumference, hypertension under treatment, daily drowsiness, snoring, and apnea during sleep [9,10]. Therefore, the STOP-Bang score was selected as an index correlated with SpO2 in relation to hypoxia during sedation, since all items can be easily confirmed in advance with preoperative anesthesia evaluation without additional monitoring devices.
As a result, the STOP-Bang score and the lowest SpO2 showed a negative correlation (coefficient = –0.774, 95% CI: –0.855 to –0.649, SE = 0.054, P < 0.001). Although there were age and BMI items in the STOP-Bang questionnaire, weak interactions were observed between oxygen saturation and actual age or BMI. The reason for this low correlation is thought to be the binary classification of these items, such as age of 50 years or older and BMI above 35 kg/m2.
Hypoxic events occurred in eight patients, hypoxia was resolved in two patients by supplying oxygen through a simple mask, but the other 6 patients resolved hypoxia through jaw thrust or manual ventilation. In 4 of these cases, the dexmedetomidine infusion rate was adjusted to half. This result is thought to have been influenced by their relatively high ages. The mean age of the hypoxic event group (69.5 ± 6.6) was higher than that of the group without events (59.8 ± 15.3)
ROC analysis was performed to check whether the STOP-Bang score is valid as a diagnostic tool for predicting hypoxia (Fig. 3 ). Consequently, the STOP-Bang score showed a stronger association with hypoxic events than the age and BMI of the patients. The cut-off value of the STOP-Bang score for predicting hypoxic events was 4 points. Previous studies by Chung et al. [13] showed a cut-off value of 3 points for the STOP-Bang score. This may be a result of the differences in the average BMI and the average neck circumference, probably due to the nationality of the patient sample. The item “B” did not apply to any patient, and item “N” applied to only one patient. In this study, the cut-off value of the BMI was 27. According to the Asia-Pacific obesity diagnosis standards, obesity is defined as a BMI of > 25, and high obesity is defined as a BMI of > 30. It seems that the standard points for BMI and neck circumference for the STOP-Bang questionnaire are quite high for most Korean patients. This is considered the reason for the maximum STOP-Bang score of 5 in this study.
In the odds ratio analysis for 8 individual items, the “Snoring,” “Observed,” and “Pressure” items were statistically significant. In particular, the “Observed” item showed a very high odds ratio of 6.0, compared with those of the other two items, which were nearly 1.4. Although it usually refers to observation by non-medical personnel, this result is consistent with the intuition that the “Observed’ item is highly correlated with OSA, and similar reports have been made in studies such as Neves Junior et al. [18].
The limitation of this study was that the oxygen mask with airway adjustments was immediately applied for patient safety when the SpO2 decreased to 92% and lasted more than 30 s or rapidly decreased to less than 90%. Therefore, it was not possible to accurately evaluate the reduction in SpO2 in the high-risk group. In addition, since there is a part in which the patients answer the questions themselves, it is difficult to calculate the correct score if there is an incorrect answer from the patient, whether intended or not.
The duration for the lowest SpO2 was not directly collected, and the times of occurrence could not be analyzed. The 5-min interval recording system did not reflect the saturation in real-time, and it was impossible to collect retrospective data. However, according to the author’s experience, most cases of hypoxia occurred nearly immediately after the loading dose was fully administered. There may be bias due to oxygen supply, and further research is needed.
In conclusion, we confirmed that the STOP-Bang was correlated with SpO2 in patients undergoing dexmedetomidine sedation in this study. This study proposes the use of the STOP-Bang score for preoperative evaluation and sedation management. The addition of the “observed” item for the pre-anesthesia evaluation may also be helpful.
SUPPLEMENTARY MATERIALS
Supplementary data including a STOP-Bang questionnaire can be found online at https://doi.org/10.17085/apm.21011
Supplementary Fig. 1.
The official version of the STOP-Bang questionnaire by Chung et al. [13] was accessed www.stopbang.ca.
Supplementary Fig. 2.
The Korean version of the STOP-Bang questionnaire form by Chung et al. [13] was accessed at www.stopbang.ca.
Notes
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
AUTHOR CONTRIBUTIONS
Conceptualization: Yusom Shin. Data curation: Minsu Yun, Sungwon Ryu, Seo Han. Formal analysis: Minsu Yun, Sungwon Ryu. Methodology: Yusom Shin. Project administration: Jiwook Kim. Investigation: Seo Han. Software: Minsu Yun. Supervision: Jiwook Kim, Yusom Shin. Writing - original draft: Minsu Yun, Yusom Shin. Writing - review & editing: Minsu Yun, Yusom Shin.
REFERENCES
1. Borgeat A, Aguirre J. Sedation and regional anesthesia. Curr Opin Anaesthesiol. 2009; 22:678–82.
2. Elkalla RS, El Mourad MB. Respiratory and hemodynamic effects of three different sedative regimens for drug induced sleep endoscopy in sleep apnea patients. A prospective randomized study. Minerva Anestesiol. 2020; 86:132–40.
3. Chang ET, Certal V, Song SA, Zaghi S, Carrasco-Llatas M, Torre C, et al. Dexmedetomidine versus propofol during drug-induced sleep endoscopy and sedation: a systematic review. Sleep Breath. 2017; 21:727–35.
4. Xu JK, Ye JY, Cao X, Zhang YH, Yuan XM, Zhao CM. [Polysomnographic comparation between dexmedetomidine-induced sleep and natural sleep]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019; 54:405–9. Chinese.
5. Amornyotin S. Sedation-related complications in gastrointestinal endoscopy. World J Gastrointest Endosc. 2013; 5:527–33.
6. Geng W, Jia D, Wang Y, Jin S, Ren Y, Liang D, et al. A prediction model for hypoxemia during routine sedation for gastrointestinal endoscopy. Clinics (Sao Paulo). 2018; 73:e513.
7. Coté GA, Hovis CE, Hovis RM, Waldbaum L, Early DS, Edmundowicz SA, et al. A screening instrument for sleep apnea predicts airway maneuvers in patients undergoing advanced endoscopic procedures. Clin Gastroenterol Hepatol. 2010; 8:660–5. e1.
8. Mathangi K, Mathews J, Mathangi CD. Assessment of perioperative difficult airway among undiagnosed obstructive sleep apnoea patients undergoing elective surgery: a prospective cohort study. Indian J Anaesth. 2018; 62:538–44.
9. Larner A, B Z. Screening for obstructive sleep apnoea using the STOPBANG questionnaire. Clin Med (Lond). 2018; 18:108–9.
10. Pearson F, Batterham AM, Cope S. The STOP-Bang Questionnaire as a screening tool for obstructive sleep apnea in pregnancy. J Clin Sleep Med. 2019; 15:705–10.
11. Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S, et al. Validation of the STOP-Bang Questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and meta-analysis. PLoS One. 2015; 10:e0143697.
12. Kim KT, Cho YW. Real-world STOPBANG: how useful is STOPBANG for sleep clinics? Sleep Breath. 2019; 23:1219–26.
13. Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016; 149:631–8.
14. Oshita H, Ito N, Senoo M, Funaishi K, Mitama Y, Okusaki K. The STOP-Bang test is useful for predicting the severity of obstructive sleep apnea. JMA J. 2020; 3:347–52.
15. Kang D, Lim C, Shim DJ, Kim H, Kim JW, Chung HJ, et al. The correlation of heart rate between natural sleep and dexmedetomidine sedation. Korean J Anesthesiol. 2019; 72:164–8.
16. Shin HJ, Kim EY, Hwang JW, Do SH, Na HS. Comparison of upper airway patency in patients with mild obstructive sleep apnea during dexmedetomidine or propofol sedation: a prospective, randomized, controlled trial. BMC Anesthesiol. 2018; 18:120.
17. Chiu HY, Chen PY, Chuang LP, Chen NH, Tu YK, Hsieh YJ, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017; 36:57–70.
18. Neves Junior JAS, Fernandes APA, Tardelli MA, Yamashita AM, Moura SMPGT, Tufik S, et al. Cutoff points in STOP-Bang questionnaire for obstructive sleep apnea. Arq Neuropsiquiatr. 2020; 78:561–9.
Fig. 2.
Scatter plots of lowest oxygen saturation during sedation and various patient factors. The results are shown for (A) STOP-Bang score (B) Age (C) Body mass index. BMI: body mass index. The results are shown for (A) STOP-Bang score (B) Age (C) Body mass index.
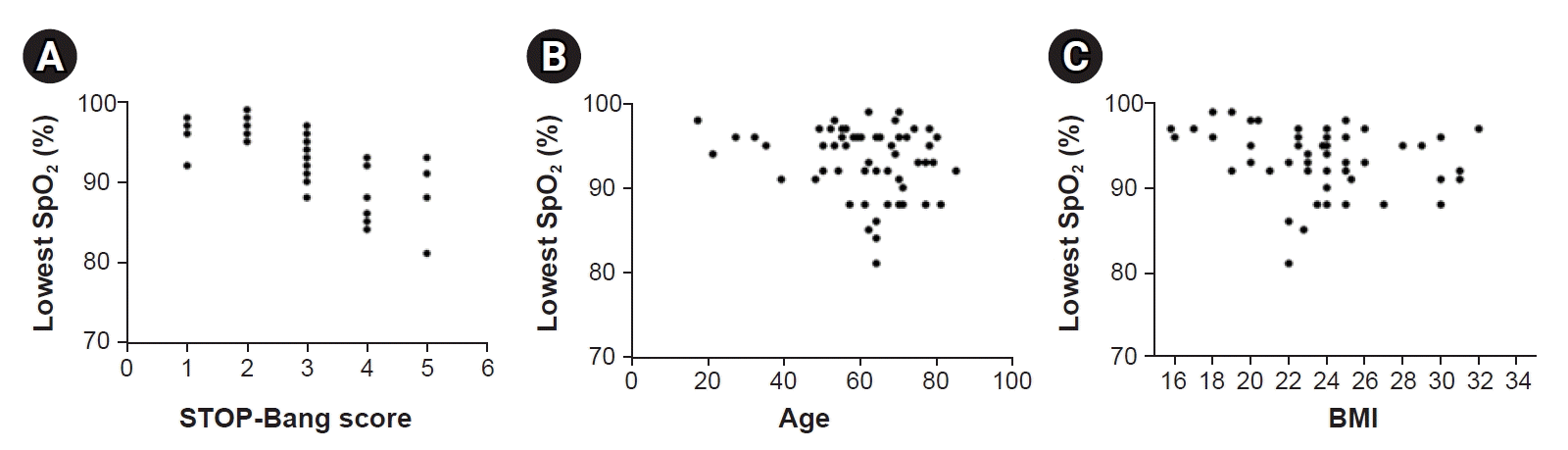
Fig. 3.
Receiver operating characteristic curves and area under the curve (AUC) for STOP-Bang score, age, and body mass index (BMI).
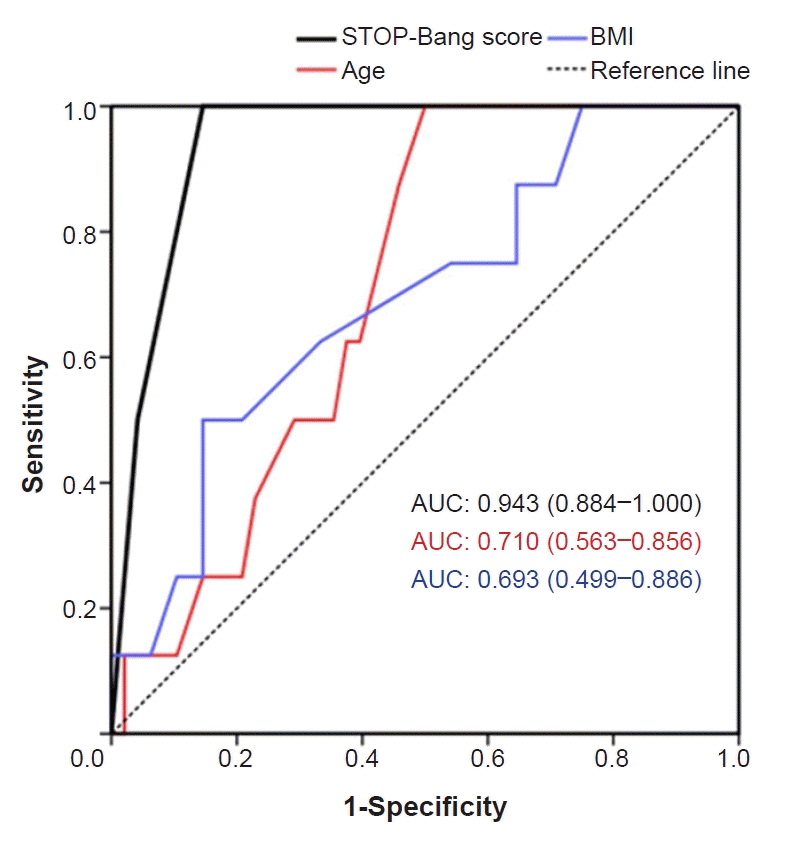
Table 1.
Demographic Characteristics of Participants (n = 56)
Table 2.
Spearman Correlation Analysis of Lowest SpO2 and STOP-Bang Score, Age, and BMI
| Items | Coefficient | 95% CI for B | SE | P value |
|---|---|---|---|---|
| STOP-Bang score | –0.774 | –0.855 to –0.649 | 0.054 | < 0.001 |
| Age | –0.201 | –0.418 to 0.032 | 0.117 | 0.137 |
| BMI | –0.288 | –0.518 to –0.013 | 0.142 | 0.031 |
Table 3.
Odds Ratios of STOP-Bang Questionnaire Items
| Questionnaire | Odds ratio | P value | 95% CI |
|---|---|---|---|
| Snoring | 1.471 | < 0.001* | 1.124 to 1.924 |
| Tired | 0.846 | 0.406 | 0.754 to 0.950 |
| Observed | 6.000 | 0.025* | 1.086 to 33.145 |
| Pressure | 1.444 | 0.001* | 1.118 to 1.866 |
| Age | 1.205 | 0.188 | 1.059 to 1.372 |
| Gender | 2.143 | 0.381 | 0.391 to 11.731 |
Table 4.
Patients Who Experienced Hypoxic Events (n = 8)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download