Abstract
Background
Tachycardia-polyuria syndrome is characterized by polyuria occurring because of tachycardia with a heart rate of ≥ 120 beats/min lasting ≥ 30 min. We report such a case occurring after swan-ganz catheterization.
Case
A 41-year-old male was scheduled for living-donor liver transplantation. After general anesthesia, atrial fibrillation occurred during swan-ganz catheterization, and polyuria developed 1 h later. During the anhepatic phase, the patient’s heart rate increased further, and cardioversion was performed. After a normal sinus rhythm was achieved, the patient’s urine output returned to normal.
Tachycardia-polyuria syndrome is characterized by the onset of polyuria after tachycardia with a heart rate (HR) of ≥ 120 beats/min persisting for at least 30 min [1]. Multiple case reports of polyuria in patients with paroxysmal supraventricular tachycardia have been published [1–3]. A reduction of antidiuretic hormone (ADH) and secretion of atrial natriuretic peptide (ANP) are known to be involved in this phenomenon [4,5]. This phenomenon more commonly affects patients with paroxysmal atrial fibrillation (a-fib) than it does those with a valvular disease or chronic heart failure [1,3,6]. Here, we report a case of a-fib during insertion of a swan-ganz catheter, followed by polyuria during living-donor liver transplantation surgery.
This case report was exempted from the need of obtaining the patient’s informed consent by the Institutional Review Board (IRB) of Asan Medical Center (no. 2021-0047).
A 41-year-old male, diagnosed with hepatocellular carcinoma, was admitted for a living-donor liver transplant. The patient (179.1 cm and 87.7 kg) was a carrier of the hepatitis B virus and had liver cirrhosis with a Child-Pugh score of 5 and a model for end-stage liver disease score of 7. The patient had no notable medical history other than hypertension. No notable findings were obtained from the chest X-ray, electrocardiogram (ECG), transthoracic echocardiography, coronary artery computed tomography, brain magnetic resonance image (MRI), lung function test, blood tests, and arterial blood gas analysis performed at the time of admission.
After arrival at the operating room, the patient’s blood pressure, ECG, and oxygen saturation (SpO2) were monitored; all his vital signs were normal. Anesthesia was induced with midazolam (5 mg), propofol (130 mg), rocuronium (100 mg), and fentanyl (100 µg). Following endotracheal intubation, anesthesia was maintained with desflurane (4–5%) and continuous infusion of fentanyl and rocuronium. We placed a catheter in the right radial artery, two catheters in the right internal jugular vein, and a catheter in the right subclavian vein to secure access to a central vein.
While inserting a swan-ganz catheter, the patient’s ECG revealed a-fib with a rapid ventricular response (RVR) (Fig. 1). We administered a beta blocker (esmolol), which did not markedly change the ECG. The patient’s vital signs at the time were as follows: arterial blood pressure, 107/71 mmHg; HR, 137 beats/min; SpO2, 99%. The arrhythmia lasted for about 9 min, and a normal heart rhythm was spontaneously achieved. To avoid further a-fib, we retracted the swan-ganz catheter to a depth of 15 cm and attempted to fully reinsert it after 20 min; however, a-fib with a RVR occurred again (Fig. 2). The patient was immediately given a beta blocker (esmolol), but the arrhythmia persisted; thus, an anti-arrhythmic agent (amiodarone) was continuously infused. The patient’s other vital signs remained stable, and transesophageal echocardiography also revealed no notable findings other than a-fib. Hence, we decided to continue the surgery while carefully monitoring the patient.
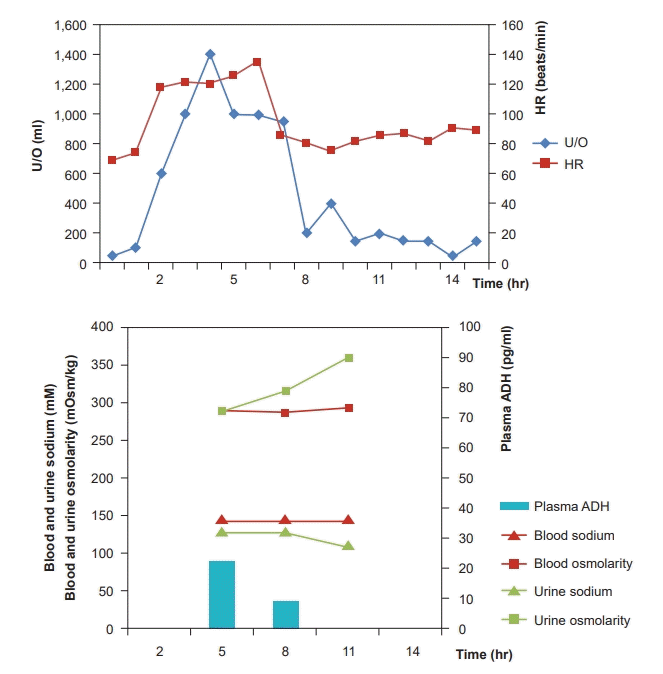
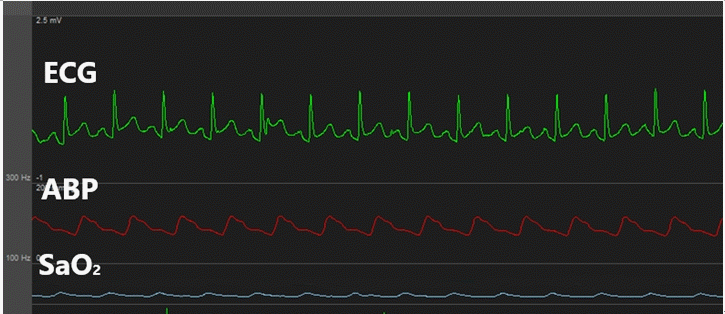
Urine output (U/O) measured as soon as the surgery commenced was abnormally high (1,000 ml/h). We suspected polyuria due to tachycardia [2,3,5-7] and, thus, performed urine analysis (U/A) and plasma ADH testing. The results indicated natriuresis (sodium clearance, 18.6 ml/min; osmolar clearance, 16.6 ml/min; 2 × urine sodium/urine osmolarity, 0.88) (Fig. 3). During the anhepatic phase, the patient’s HR increased further and exhibited hemodynamic instability; hence, direct current (DC) cardioversion (50 J) was performed, after which a normal rhythm was achieved (Fig. 4). U/O measured one hour later was markedly decreased and natriuresis was reduced (sodium clearance, 3.7 ml/min; osmolar clearance, 3.6 ml/min; 2 × urine sodium/urine osmolarity, 0.81) (Fig. 3).
The surgery proceeded without notable problems, after which the patient was transferred to the intensive care unit (ICU). The patient’s ECG revealed normal rhythms until the end of surgery. At the ICU, the patient exhibited a normal U/O and ECG, with stable vital signs, and, after being transferred to a general ward, the patient was discharged without any further problems.
We reported a case of tachycardia-polyuria syndrome that occurred during insertion of a swan-ganz catheter in a liver transplant patient.
According to Wood [1] and Luria et al. [3], tachycardia-polyuria syndrome is defined as polyuria that occurs in approximately half of patients with paroxysmal supraventricular arrhythmias faster than 110 beats/min lasting for ≥ 20 min in the absence of left ventricular failure and stenotic valvular lesions, and there are no clear diagnostic criteria for this syndrome. ADH and ANP are known to be potentially involved in the mechanism underlying tachycardia-polyuria syndrome [2,4,5,7,8]. In addition to receiving blood into the heart and releasing it into the ventricles, the atria also serve the function of detecting and regulating the intravenous volume. This process, which begins with the atrial receptors, is known to involve neuronal and hormonal networks such as ADH and ANP [4]. An elevation of venous return in the body leads to atrial distention, which stimulates the atrial receptors [9]. These receptors depolarize the afferent vagal nerve to decrease the efferent sympathetic and vasomotor tones of the renal nerve, and to reduce ADH activity, thereby inducing diuresis [6].
Polyuria refers to an abnormally high output of diluted urine (i.e., ≥ 3 L/24 h or ≥ 40–50 ml/kg/24 h). It can be caused by a variety of factors, and these conditions are collectively referred to as polyuria-polydipsia syndrome. The causes can be broadly classified into three factors: central diabetes insipidus (CDI), nephrogenic diabetes insipidus (NDI), and primary polydipsia. Because the causes and treatments for each of these factors differ, differential diagnosis is crucial [10]. The patient in this case had no abnormal preoperative brain MRI findings and had no history of endocrine diseases such as diabetes mellitus or renal diseases. Further, the patient had a normal electrolyte balance during surgery, and none of the medications administered intraoperatively were known to induce polyuria. Therefore, we could eliminate CDI and NDI from the diagnosis. The patient developed polyuria during induction of anesthesia, allowing us to eliminate volume overload. In essence, the patient’s polyuria seemed related to the iatrogenic a-fib that occurred during insertion of the swan-ganz catheter during induction of anesthesia.
It has been reported that paroxysmal a-fib induces atrial pressure and atrial volume expansion in canines under anesthesia [11]. Similarly, the a-fib observed in our patient is believed to have induced atrial volume expansion and, consequently, atrial wall distention, which would have led to a suppression of ADH and, thereby, caused diuresis. However, contrary to the mechanism of tachycardia-polyuria syndrome as it is currently understood, our patient had a higher-than-normal plasma ADH concentration.
ADH is known to regulate water absorption through the expression of aquaporin-2 on the V2 receptors of the collecting duct, and its production increases in response to increased plasma osmolality, reduced atrial pressure, and reduced intravascular volume [12]. Fuji et al. [8] reported that ADH may be increased to suppress water diuresis caused by supraventricular tachycardia, and that elevated ADH levels increased renal prostaglandin E2, thereby inducing natriuresis independently from ANP. Thus, the elevated ADH in our case seems to be a compensation mechanism to counteract the reduction of intravascular volume caused by polyuria, as opposed to being the cause of polyuria.
ANP, which serves an essential role in tachycardia-polyuria syndrome, is a cardiac hormone secreted as a result of stretching of the atrial cardiocytes [13], and elevated atrial pressure is an important factor involved in its production [5]. ANP regulates the intravascular volume by blocking renin, angiotensin, and aldosterone secretion to induce natriuresis and diuresis [14]. Kinney et al. [2] reported that U/O is increased in tachycardia-polyuria syndrome as a result of natriuresis through sodium clearance, osmolar clearance, the ratio of sodium to osmolarity in urine (2 × urine sodium / urine osmolarity), and that sodium accounts for a high percentage of the excreted solutes. In the present case, U/A performed after the onset of tachycardia indicated natriuresis. This suggests that sodium excretion was the cause of the observed diuresis [2].
One hour after achieving a normal rhythm, test results confirmed that natriuresis was reduced and, consequently, ANP levels would have been lowered [4,5]. Further, an animal study revealed that atrial pressure increased by 81 ± 19% and atrial diameter by 21 ± 5% during a-fib [11]. Taken together, increased ANP seems to be the main mechanism of tachycardia and polyuria accompanied with natriuresis. However, the fact that we did not measure ANP concentration is a limitation to our report.
The Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists published a practice advisory for the management of perioperative a-fib in patients undergoing cardiac surgery, with several recommendations [15]. According to the practice advisory, perioperative a-fib should be treated with non-dihydropyridine calcium channel blockers or beta blockers (strength of recommendation = 1). If the patient is hemodynamically unstable, they should be treated with electrical or chemical (e.g., amiodarone) cardioversion (strength of recommendation = 1). Moreover, amiodarone is recommended (strength of recommendation = 2a) to prevent a-fib after cardiac surgery. However, when unresponsive tachycardia occurs, as in the present case, DC cardioversion may be necessary to achieve a normal sinus rhythm.
The present case highlighted the possibility that iatrogenic a-fib occurring during insertion of a swan-ganz catheter may cause polyuria. Polyuria during surgery has a great influence on the patient's volume management. Therefore, an anesthesiologist must be familiar with the discrimination and treatment about perioperative polyuria. Notably, if atrial fibrillation is the cause of polyuria, DC cardioversion could be a way to stop polyuria if the drug does not respond.
Notes
REFERENCES
1. Wood P. Polyuria in paroxysmal tachycardia and paroxysmal atrial flutter and fibrillation. Br Heart J. 1963; 25:273–82.
2. Kinney MJ, Stein RM, DiScala VA. The polyuria of paroxysmal atrial tachycardia. Circulation. 1974; 50:429–35.
3. Luria MH, Adelson EI, Lochaya S. Paroxysmal tachycardia with polyuria. Ann Intern Med. 1966; 65:461–70.
4. Hirata Y, Nozaki A, Toda I, Murakawa Y, Sugimoto T, Matsuoka H, et al. Plasma concentration of atrial natriuretic polypeptide in patients with atrial tachycardia. Jpn Heart J. 1987; 28:53–61.
5. Nilsson G, Pettersson A, Hedner J, Hedner T. Increased plasma levels of atrial natriuretic peptide (ANP) in patients with paroxysmal supraventricular tachyarrhythmias. Acta Med Scand. 1987; 221:15–21.
6. Zullo MA. Atrial regulation of intravascular volume: observations on the tachycardia-polyuria syndrome. Am Heart J. 1991; 122(1 Pt 1):188–94.
7. Canepa-Anson R, Williams M, Marshall J, Mitsuoka T, Lightman S, Sutton R. Mechanism of polyuria and natriuresis in atrioventricular nodal tachycardia. Br Med J (Clin Res Ed). 1984; 289:866–8.
8. Fujii T, Kojima S, Imanishi M, Ohe T, Omae T. Different mechanisms of polyuria and natriuresis associated with paroxysmal supraventricular tachycardia. Am J Cardiol. 1991; 68:343–8.
9. Paintal AS. A study of right and left atrial receptors. J Physiol. 1953; 120:596–610.
10. Nigro N, Grossmann M, Chiang C, Inder WJ. Polyuria-polydipsia syndrome: a diagnostic challenge. Intern Med J. 2018; 48:244–53.
11. White CW, Kerber RE, Weiss HR, Marcus ML. The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ Res. 1982; 51:205–15.
12. Treschan TA, Peters J. The vasopressin system: physiology and clinical strategies. Anesthesiology. 2006; 105:599–612; quiz 639-40.
13. Cantin M, Genest J. The heart and the atrial natriuretic factor. Endocr Rev. 1985; 6:107–27.
14. Laragh JH. The endocrine control of blood volume, blood pressure and sodium balance: atrial hormone and renin system interactions. J Hypertens Suppl. 1986; 4:S143–56.
15. O'Brien B, Burrage PS, Ngai JY, Prutkin JM, Huang CC, Xu X, et al. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists practice advisory for the management of perioperative atrial fibrillation in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2019; 33:12–26.
Fig. 1.
This is atrial fibrillation occurred when swan-ganz catheter was inserted. ECG: electrocardiogram, ABP: arterial blood pressure.
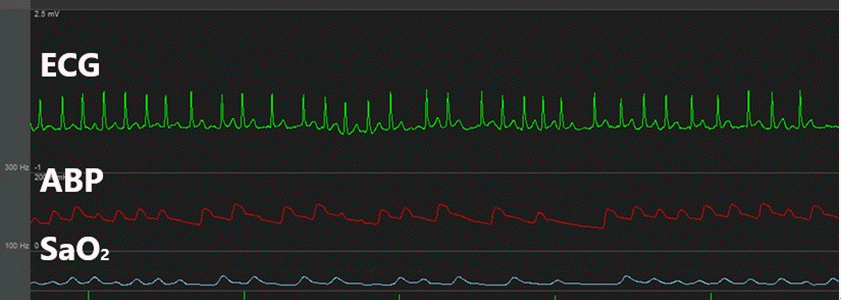
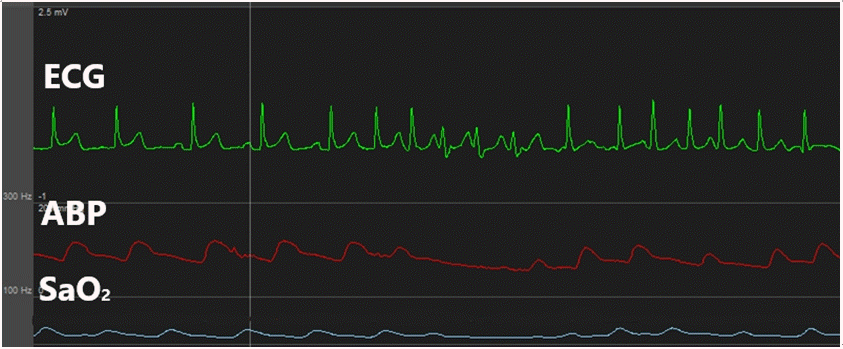
Fig. 2.
This is atrial fibrillation occurred when swan-ganz catheter was re-inserted. ECG: electrocardiogram, ABP: arterial blood pressure.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download