Abstract
Background
Postpartum hemorrhage is the leading cause of maternal mortality. Oxytocin being the most popular uterotonic agent, has been routinely administered after both vaginal delivery and cesarean section. Carbetocin is a newer uterotonic agent and provides the benefit of a longer duration of action without additional administration post-delivery.
Methods
We recruited 34 women undergoing elective cesarean section under spinal anesthesia. All patient was received spinal anesthesia using 0.5% hyperbaric Marcaine 8–10 mg in conjugation with fentanyl 20 μg in the left lateral decubitus position. Hartmann’s solution 10–15 ml/kg was administered before carbetocin. The operation started as soon as sensory block at level T4–T6 was confirmed. A non-invasive hemodynamic monitoring cuff (Finometer®) was attached to the patient’s finger soon after the induction of spinal anesthesia. Using the Finometer, we recorded the heart rate and mean arterial pressure at every 15 s, starting from 15 s before the administration of carbetocin to 5 min after. After the removal of the placenta, the bolus group was administered intravenous bolus injection of carbetocin 100 μg and the infusion group was administered carbetocin 100 μg diluted in 50 ml normal saline, over 5 min using an infusion pump.
Postpartum hemorrhage (PPH) is associated with nearly one quarter of all maternal deaths globally. It is also the leading cause of maternal mortality in most low-income countries [1,2]. Uterine atony is the most common cause of PPH [3]. The World Health Organization (WHO) recommends active management of the third stage of labor and use of uterotonics for the prevention of PPH during vaginal delivery and cesarean section [2].
Oxytocin being the most popular uterotonic agent, has been routinely administered after both vaginal delivery and cesarean section [4]. However, it is associated with dose-related side effects, including hypotension, tachycardia, nausea and vomiting [5]. Other reported side effects include pulmonary edema due to the antidiuretic effect of oxytocin [6,7]. Thomas et al. [8] compared hemodynamic change between a group that was administered oxytocin by bolus injection and another group that was administered oxytocin by infusion. They concluded that the infusion group had lesser hemodynamic changes than the bolus group.
Carbetocin is a newer uterotonic agent and a long-acting synthetic octapeptide analogue of oxytocin with oxytocin receptor agonist properties. Because carbetocin has a 4–10 fold longer elimination half-life than that of oxytocin, it provides the benefit of longer duration of oxytocic action without additional administration in post-delivery [4]. Although the difference between carbetocin and oxytocin in terms of hemodynamic changes, postpartum blood loss and incidence of side effects is not statistically significant [4,9], carbetocin is superior to oxytocin in terms of additional dosing and cost-effectiveness [10].
There is no definite consensus about how to administer carbetocin. We compared the hemodynamic effects of carbetocin when administered as an intravenous bolus and as an infusion over 5 minutes using a non-invasive hemodynamic monitoring device.
This study was approved by the Hospital Ethics Committee (Dongguk University Ilsan Hospital Institutional Review Board, registration no. 2012-77). The study was registered with Clinical Research Information Service, Korea (https://cris.nih.go.kr, registration no. KCT0000715). We recruited 34 women undergoing elective cesarean section under spinal anesthesia. The patients were 37 weeks or more into their pregnancy and were classified as Class 1 or 2 according to the American Society of Anesthesiologists physical status classification system. The patients who had preeclampsia, placenta previa, other diseases affecting hemodynamic change and past medical history of hypertension or diabetes were excluded. The written consent was given by all subjects before operation. The subjects were randomly divided into two groups. Prior to enrolling patients, group assignments were placed in 34 opaque envelopes. These envelopes were mixed and placed in a container. Before each patient entered the operating room, the anesthesia provider randomly selected one of these opaque concealed envelopes to ensure group randomization.
None of patients were premedicated. Non-invasive blood pressure monitoring device, pulse oximeter and electrocardiograph were attached to the patient as soon as they arrived in the operating room. All patients received spinal anesthesia at L3/4 or L4/5 level using 0.5% hyperbaric Marcaine 8–10 mg and fentanyl 20 μg in the left lateral decubitus position. Hartmann’s solution 10–15 ml/kg was administered before administration of carbetocin. The operation started as soon as the sensory block at level T4–T6 was confirmed.
A non-invasive hemodynamic monitoring cuff (Finometer®, Finapres medical system, Netherlands) was attached to the patient’s finger soon after the induction of spinal anesthesia. Using the Finometer, heart rate (HR) and mean arterial pressure (MAP) was recorded at every 15 s starting from 15 s before the administration of carbetocin to 5 min after.
After the removal of placenta, the bolus group received an intravenous bolus injection of carbetocin 100 μg and the infusion group received 100 μg carbetocin diluted in 50 ml normal saline, over 5 min using an infusion pump (Orchestra® Module DPS, Fresenius Kabi, France). We defined hypotension as less than 20% of the preoperative value or below 80 mmHg systolic blood pressure. Hypotension was treated with ephedrine 5 mg boluses. If ephedrine was used during the study period, that patient would be excluded in study.
The primary endpoint was the mean difference in HR and MAP between study groups at each time point. The secondary endpoints were estimated blood loss (EBL), the presence of postoperative hemorrhage and the requirement for additional administration of uterotonic agent.
A MAP difference of 10 mm Hg between the two groups was considered clinically significant. According to the previous study reporting a standard deviation of 8.7 mmHg [8], a sample of 17 patients per group was required with α error of 0.05 and power of 90%.
Demographic data variables including age, height, weight, and body mass index, ephedrine dose, and operation time were analyzed using unpaired t-tests. The primary endpoints were analyzed at each time point using unpaired t-tests. EBL of each group was compared using the Mann-Whitney test. Other secondary endpoints were compared using Fisher’s exact test. All statistical analysis were performed using R software version 3.3.2 (R Core Team [2016]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Austria. URL: https://www.R-project.org/).
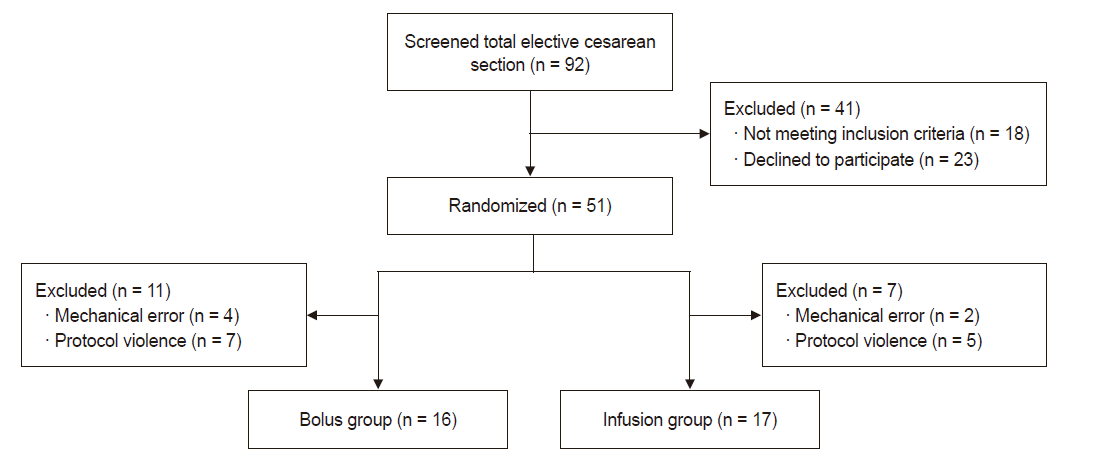
The study was conducted over a continuous period of 1 year. A total 34 patients were recruited. No patient was excluded except one whose data were missing (Fig. 1). The patient characteristics are shown in Table 1. No statistical difference was observed between the two groups in demographic data. None of patient received ephedrine during the study period and no difference in the total dose of ephedrine used between the two groups. Baseline HR and MAP were not statistically different either (Table 2).
Moreover, there was no statistical difference in the mean HR and MAP between the two groups (Figs. 2, 3). In both groups, we found a rapid decrease of MAP until 30 s after the administration of carbetocin regardless of the method of administration. Then, the trends showed a gradual increase. After 300 s, the mean MAP of the bolus group was 88.73 mmHg and that of the infusion group was 86.76 mmHg. The mean MAP of the bolus group was higher than that of the infusion group from 105 to 300 s, but there was no statistically significant difference at any time point.
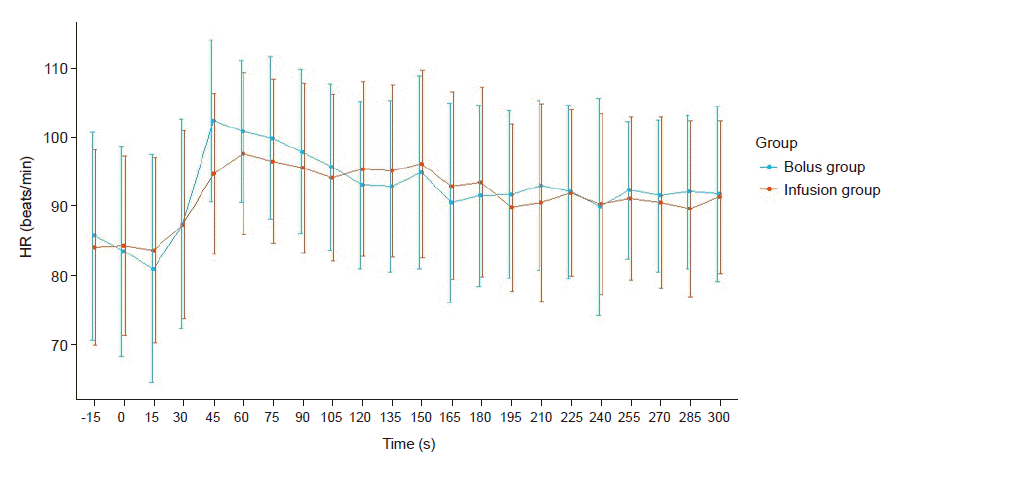
In both groups, we found a rapid increase of HR until 45 s after the administration of carbetocin. Then, the trends showed a gradual decrease till the end of the study. After 300 s, the mean HR of the bolus group was 92.35 beats/min and that of the infusion group was 91.71 beats/min. Again, the difference between the two groups at any time point was not statistically significant. We also investigated EBL, additional administration of uterotonic agent in both groups and the incidence of postoperative intervention such as uterine artery embolization or hysterectomy (Table 3). There were no significant differences between the two groups. Only one patient received postoperative uterine artery embolization and transfusion during PPH.
Our study showed that the method of administration of carbetocin does not influence its hemodynamic effects in cesarean section and does not affect additional uterotonic agent use or incidence of postoperative intervention such as uterine artery embolization or hysterectomy.
Carbetocin is a long-acting synthetic nonapeptide analogue of oxytocin with agonist properties. It can be administered intravenously as a single dose immediately following vaginal delivery or cesarean section, to prevent uterine atony and postpartum hemorrhage. Similar to oxytocin, it selectively binds to oxytocin receptors present on the smooth musculature of the uterus, resulting in rhythmic contractions of the uterus, increased frequency of existing contractions, and increased uterine tone [11].
The Finometer® measures finger blood pressure noninvasively on a beat-to-beat basis and gives waveform measurements similar to those of intra-arterial recordings. One disadvantage is the measured value changes with the movement of the patient’s finger or posture. However, its use is comfortable for conscious patients because it is non-invasive and the correlation can be easily calculated by measuring the brachial cuff pressure and hydrostatic height of the finger [12].
According to WHO guidelines for prevention of PPH, oxytocin (10 IU, intravenous or intramuscular) is the uterotonic agent of choice during the third stage of labor and cesarean section. Although the use of carbetocin as an additional uterotonic agent has been recommended, according to WHO guidelines, carbetocin has no effect on the incidence of major obstetric hemorrhage and is more expensive than oxytocin [2].
Recent studies have suggested that carbetocin is superior to oxytocin in cost-effectiveness and prevention of PPH. van der Nelson et al. [10] compared the cost-effectiveness of carbetocin used for the prevention of PPH in cesarean section to that of oxytocin. They analyzed 1,500 patients receiving carbetocin or oxytocin in cesarean section and compared the costs for additional drugs and blood products used, which in turn depends on the incidence and severity of PPH. According to this study, in UK, the use of carbetocin instead of oxytocin reduced the costs by EUR 27,518. Jin et al. [13] examined 12 randomized controlled studies and found that carbetocin lowered the requirement for additional uterotonic agents and uterine massage in women who underwent cesarean section. However, their analysis could not detect a significant difference in PPH, EBL or adverse effects.
In the case of oxytocin, several reports have shown an association between an infusion of diluted oxytocin and milder hemodynamic changes compared to bolus administration. Thomas et al. [8] compared the effects of oxytocin administered as an intravenous bolus and as an infusion for 5 min in 30 women undergoing cesarean section. They reported marked cardiovascular changes in the bolus group. Kim et al. [14] also reported a similar result with regards to cardiovascular changes with a difference in the uterine tone and EBL.
Our study showed that the method of administration does not have a significant effect on the patient’s hemodynamics in the case of carbetocin. Dell-Kuster et al. [15] investigated obstetric and hemodynamic differences between carbetocin bolus and infusion. They found that the administration of carbetocin as a short infusion does not compromise the uterine tone and that two methods have similar cardiovascular effects and vasoconstrictor dose requirements.
Intraoperative hypotension during spinal anesthesia for elective cesarean delivery are frequent above 70%, when pharmacological prophylaxis is not used [16]. However, hypotension is prevented or improved by several methods such as prevention of aorto-caval compression, use of vasopressors and intravenous fluid loading. In our study, no occurrence of hypotension during the investigation period might be a result of a mere coincidence. But, we thought that sufficient fluid loading, the exclusion of emergency surgery and other effective treatment for preventing hypotension contributed to that result.
There were several limitations to this study. First, the calculated EBL of both groups were statistically similar, but there may have been a significant difference in the actual blood loss which is difficult to quantify [17,18]. A recent study recommends a colorimetric system for more accurate measurement of the amount of blood loss. Although we could not apply a colorimetric system, the difference in the actual blood loss between the two groups in our study is not expected to be large since the calculated EBL in the above mentioned study was similar to that of our study [18]. In the future, we aim to develop and optimize methods for the accurate measurement of blood loss and intravascular volume status. Furthermore, this study does not investigate postoperative hemodynamic change. Although there were no differences between the two groups in terms of additional uterotonic drug use or postoperative complications, it is necessary to measure postoperative vital sign for investigating the precise hemodynamic effect of carbetocin
In conclusion, the administration method of carbetocin does not influence the hemodynamic changes. In addition, the administration method has no effect on postoperative complications, additional administration of uterotonic agents or blood loss.
REFERENCES
1. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2:e323–33.
2. World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization;2012. p. 41.
3. Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009; 9:55.
4. Meshykhi LS, Nel MR, Lucas DN. The role of carbetocin in the prevention and management of postpartum haemorrhage. Int J Obstet Anesth. 2016; 28:61–9.
5. Petersson M. Cardiovascular effects of oxytocin. Prog Brain Res. 2002; 139:281–8.
6. Shahin J, Guharoy SR. Pulmonary edema possibly developing secondary to the intravenous administration of oxytocin. Vet Hum Toxicol. 1991; 33:587–8.
7. Ghai B, Vayjnath AM, Lal S. Acute pulmonary oedema following oxytocin administration: a life threatening complication. J Indian Med Assoc. 2006; 104:261–2.
8. Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth. 2007; 98:116–9.
9. Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG. 2011; 118:1349–56.
10. van der Nelson HA, Draycott T, Siassakos D, Yau CWH, Hatswell AJ. Carbetocin versus oxytocin for prevention of post-partum haemorrhage at caesarean section in the United Kingdom: an economic impact analysis. Eur J Obstet Gynecol Reprod Biol. 2017; 210:286–91.
11. Sweeney G, Holbrook AM, Levine M, Yip M, Alfredsson K, Cappi S, et al. Pharmacokinetics of carbetocin, a long-acting oxytocin analogue, in nonpregnant women. Curr Ther Res Clin Exp. 1990; 47:528–40.
12. Schutte AE, Huisman HW, van Rooyen JM, Malan NT, Schutte R. Validation of the Finometer device for measurement of blood pressure in black women. J Hum Hypertens. 2004; 18:79–84.
13. Jin B, Du Y, Zhang F, Zhang K, Wang L, Cui L. Carbetocin for the prevention of postpartum hemorrhage: a systematic review and meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med. 2016; 29:400–7.
14. Kim TS, Bae JS, Park JM, Kang SK. Hemodynamic effects of continuous intravenous injection and bolus plus continuous intravenous injection of oxytocin in Cesarean section. Korean J Anesthesiol. 2011; 61:482–7.
15. Dell-Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of carbetocin given as an intravenous bolus compared with short infusion for Caesarean section - double-blind, double-dummy, randomized controlled non-inferiority trial. Br J Anaesth. 2017; 118:772–80.
16. Mercier FJ, Augè M, Hoffmann C, Fischer C, Le Gouez A. Maternal hypotension during spinal anesthesia for caesarean delivery. Minerva Anestesiol. 2013; 79:62–73.
17. Lopez-Picado A, Albinarrate A, Barrachina B. Determination of perioperative blood loss: accuracy or approximation? Anesth Analg. 2017; 125:280–6.
18. Doctorvaladan SV, Jelks AT, Hsieh EW, Thurer RL, Zakowski MI, Lagrew DC. Accuracy of blood loss measurement during Cesarean delivery. AJP Rep. 2017; 7:e93–100.
Fig. 3.
The changes of mean arterial pressure (MAP) in two groups. Values are presented as mean ± SD.
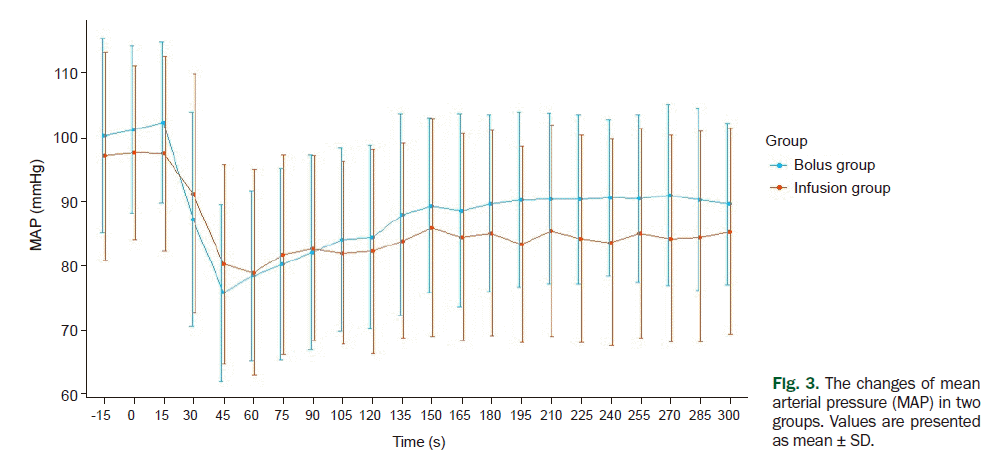
Table 1.
The Demographic Data of Two Groups
Table 2.
Initial Heart Rate and Mean Arterial Pressure of Two Groups
| Initial hemodynamic variables | Bolus group | Infusion group |
|---|---|---|
| Initial HR (beats/min) | 83.6 ± 15.3 | 84.4 ± 13.0 |
| Initial MAP (mmHg) | 101.2 ± 12.9 | 97.6 ± 13.5 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download