INTRODUCTION
MATERIALS AND METHODS
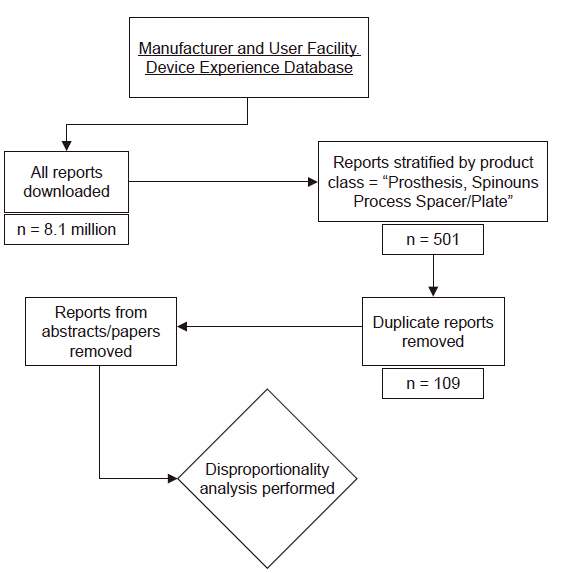
MAUDE database
Data processing
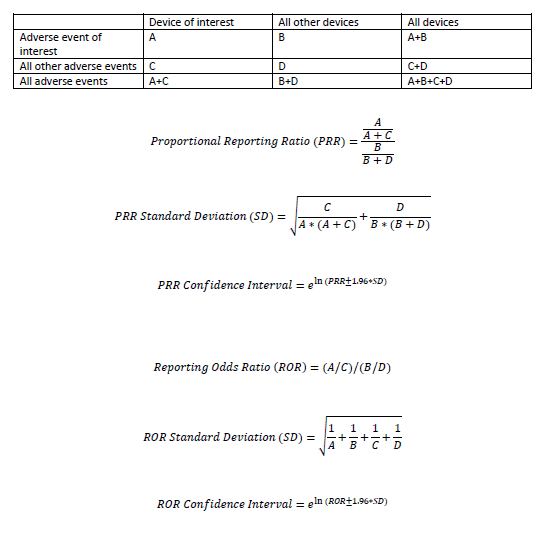
Disproportionality analysis
1. Lower limit of the 95% confidence interval (CI) for ROR greater than one [5]
2. PRR is greater than or equal to 2, chi-squared is greater than or equal to 4, and the number of device/drug to adverse event reports greater than or equal to 3, a statistically significant signal is found [6]
3. Lower limit of the 95% confidence interval for IC (IC025) greater than [7]
RESULTS
Table 1.
| Adverse events | Count (n) | ROR (95% CI) | PRR (95% CI) | Chi-squared with Yates correction |
|---|---|---|---|---|
| Allergic | ||||
| Coflex | 0 | - | - | - |
| Vertiflex | 3 | 9.15 (2.90–28.82)* | 8.92 (2.92–27.23)† | 13.96 |
| X-Stop | 3 | 3.97 (1.27–12.39)* | 3.93 (1.28–12.11) | 3.97 |
| CSF leak | ||||
| Coflex | 1 | 60.09 (8.25–437.77)* | 58.57 (8.46–405.56) | 13.66 |
| Vertiflex | 0 | - | - | - |
| X-Stop | 2 | 18.80 (4.67–75.62)* | 18.65 (4.69–74.19) | 18.09 |
| Fracture | ||||
| Coflex | 8 | 10.18 (4.68–22.16)* | 8.30 (4.47–15.40)† | 45.45 |
| Vertiflex | 15 | 6.37 (3.69–10.98)* | 5.62 (3.51–8.99)† | 53.77 |
| X-Stop | 46 | 9.12 (6.62–12.57)* | 7.60 (5.86–9.86)† | 263.49 |
| Infection | ||||
| Coflex | 5 | 3.61 (1.41–9.22)* | 3.27 (1.44–7.42)† | 6.01 |
| Vertiflex | 7 | 1.70 (0.79–3.66) | 1.65 (0.81–3.39) | 1.26 |
| X-Stop | 14 | 1.49 (0.87–2.55) | 1.46 (0.88–2.43) | 1.65 |
| Malfunction | ||||
| Coflex | 3 | 0.05 (0.01–0.15) | 0.12 (0.04–0.35) | 53.62 |
| Vertiflex | 11 | 0.06 (0.03–0.11) | 0.16 (0.09–0.27) | 139.74 |
| X-Stop | 17 | 0.04 (0.02–0.07) | 0.11 (0.07–0.17) | 359.26 |
| Migration | ||||
| Coflex | 8 | 20.83 (9.57–45.32)* | 16.76 (9.04–31.09)† | 104.61 |
| Vertiflex | 23 | 21.84 (13.78–34.63)* | 17.40 (12.11–25.01)† | 343.54 |
| X-Stop | 36 | 13.91 (9.76–19.81)* | 12.01 (8.88–16.24)† | 356.67 |
| Pain/worsening symptoms | ||||
| Coflex | 13 | 4.78 (2.46–9.30)* | 3.52 (2.26–5.49)† | 23.19 |
| Vertiflex | 27 | 3.19 (2.06–4.93)* | 2.64 (1.90–3.66)† | 28.59 |
| X-Stop | 94 | 5.95 (4.60–7.70)* | 4.05 (3.46–4.75)† | 235.27 |
Table 2.
MAUDE: Manufacturer and User Facility Device Experience, CSF: cerebrospinal fluid, CI: confidence interval, ROR: reporting odds ratio, PRR: proportional reporting ratio, IC: information component. Coflex (Paradigm Spine LLC, USA). Vertiflex (Boston Scientific, USA). X-Stop (Medtronic Inc., USA). Criterion 1: ROR Lower 95% CI greater than 1. Criterion 2: PRR greater than or equal to 2 and chi-squared greater than or equal to 4 and the number of device/drug to adverse event reports greater than or equal to 3. Criterion 3: IC025 greater than zero.
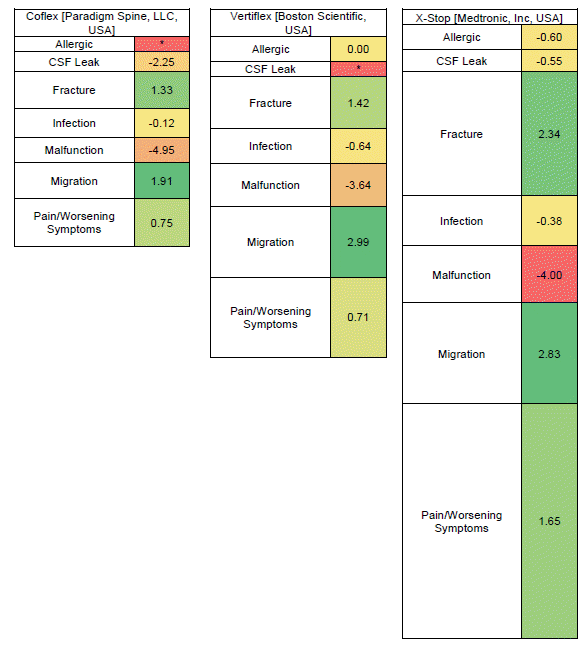 | Fig. 3.Heat Map for each of the spinous process spacers/plates and adverse event combinations. IC025 for each device by adverse event is displayed in the center of the box. Color is coded by IC025 with values greater than 0 being green and those less than 0 being yellow/red. The size of each box is representative of the relative number of adverse events for that device and adverse event combination. CSF: cerebrospinal fluid, IC: information component. *These device and adverse event combinations had 0 reports and are shown with the smallest box size. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download