1. Chaudhry A, Kamali A, Herzka DA, Wang KC, Carrino JA, Blitz AM. Detection of the stellate and thoracic sympathetic chain ganglia with high-resolution 3D-CISS MR imaging. AJNR Am J Neuroradiol. 2018; 39:1550–4.
2. Elias M. Cervical sympathetic and stellate ganglion blocks. Pain Physician. 2000; 3:294–304.
3. Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr Physiol. 2016; 6:1239–78.
4. Krumova EK, Gussone C, Regeniter S, Westermann A, Zenz M, Maier C. Are sympathetic blocks useful for diagnostic purposes? Reg Anesth Pain Med. 2011; 36:560–7.
5. Gibbs GF, Drummond PD, Finch PM, Phillips JK. Unravelling the pathophysiology of complex regional pain syndrome: focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008; 35:717–24.
6. Sommer C, Leinders M, Üçeyler N. Inflammation in the pathophysiology of neuropathic pain. Pain. 2018; 159:595–602.
7. Park J, Lee YJ, Kim ED. Clinical effects of pulsed radiofrequency to the thoracic sympathetic ganglion versus the cervical sympathetic chain in patients with upper-extremity complex regional pain syndrome: a retrospective analysis. Medicine (Baltimore). 2019; 98:e14282.
8. Zhu X, Kohan LR, Morris JD, Hamill-Ruth RJ. Sympathetic blocks for complex regional pain syndrome: a survey of pain physicians. Reg Anesth Pain Med;2019; doi: 10.1136/rapm-2019-100418 [Epub ahead of print].
9. Rocha Rde O, Teixeira MJ, Yeng LT, Cantara MG, Faria VG, Liggieri V, et al. Thoracic sympathetic block for the treatment of complex regional pain syndrome type I: a double-blind randomized controlled study. Pain. 2014; 155:2274–81.
10. Murata Y, Takahashi K, Yamagata M, Takahashi Y, Shimada Y, Moriya H. Variations in the number and position of human lumbar sympathetic ganglia and rami communicantes. Clin Anat. 2003; 16:108–13.
11. Hong JH, Kim AR, Lee MY, Kim YC, Oh MJ. A prospective evaluation of psoas muscle and intravascular injection in lumbar sympathetic ganglion block. Anesth Analg. 2010; 111:802–7.
12. Schiller Y. The anatomy and physiology of the sympathetic innervation to the upper limbs. Clin Auton Res. 2003; 13 Suppl 1:I2–5.
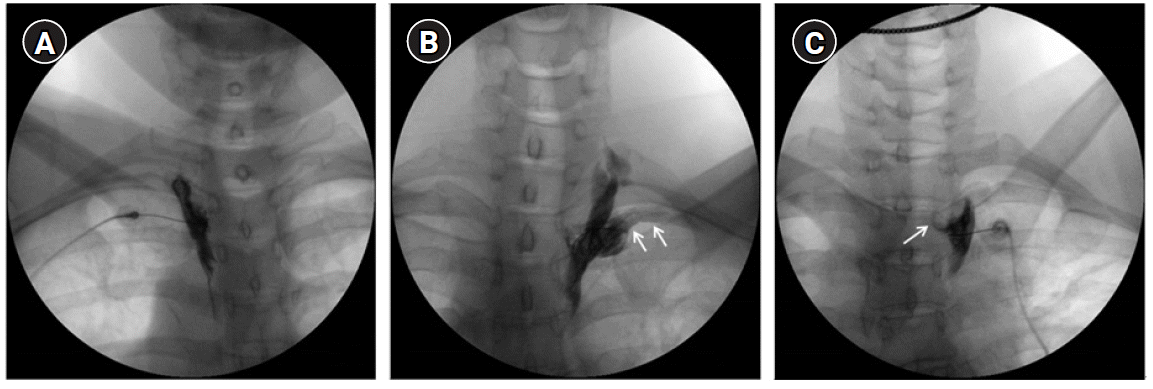
13. Kim WH, Lee CJ, Kim TH, Shin BS, Sim WS. The optimal oblique angle of fluoroscope for thoracic sympathetic ganglion block. Clin Auton Res. 2011; 21:89–96.
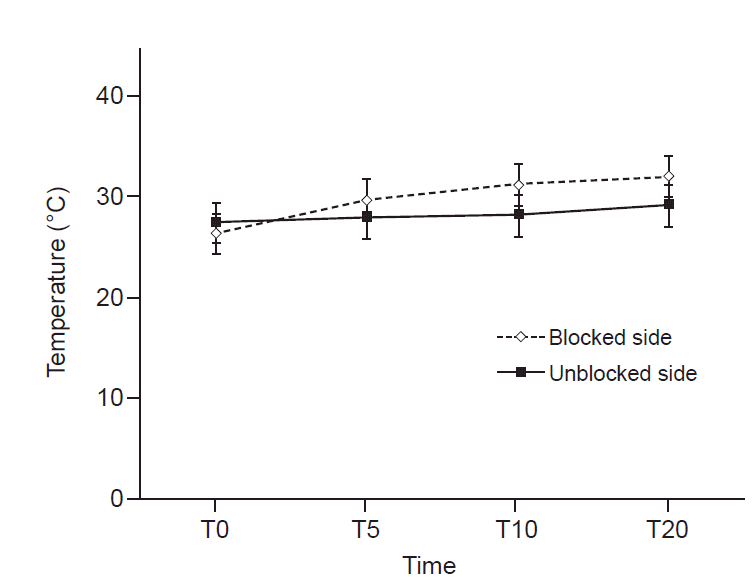
14. Schürmann M, Gradl G, Wizgal I, Tutic M, Moser C, Azad S, et al. Clinical and physiologic evaluation of stellate ganglion blockade for complex regional pain syndrome type I. Clin J Pain. 2001; 17:94–100.
15. Cho HM, Lee DY, Sung SW. Anatomical variations of rami communicantes in the upper thoracic sympathetic trunk. Eur J Cardiothorac Surg. 2005; 27:320–4.
16. Akil A, Semik M, Fischer S. Efficacy of miniuniportal video-assisted thoracoscopic selective sympathectomy (ramicotomy) for the treatment of severe palmar and axillar hyperhidrosis. Thorac Cardiovasc Surg. 2019; 67:415–9.
17. Brock M, Frangakis C, Georgiades CS. CT-guided, percutaneous ethanol sympatholysis for primary hyperhidrosis. Cardiovasc Intervent Radiol. 2018; 41:477–82.
18. Ramsaroop L, Partab P, Singh B, Satyapal KS. Thoracic origin of a sympathetic supply to the upper limb: the 'nerve of Kuntz' revisited. J Anat. 2001; 199(Pt 6):675–82.
19. Choi E, Nahm FS, Lee PB. Sympathetic block as a new treatment for lymphedema. Pain Physician. 2015; 18:365–72.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download