Abstract
Background
Fontan-associated liver disease (FALD) is a hepatic disorder caused by hemodynamic changes and systemic venous congestion following the Fontan procedure. FALD includes liver cirrhosis and hepatocellular carcinoma (HCC), both of which may require liver transplantation (LT). However, the Fontan circulation, characterized by elevated central venous pressure and reduced cardiac output, is a challenging issue for surgeons and anesthesiologists.
Case
We report a living-donor LT for the treatment of HCC. The patient was a 24-year-old male who underwent the Fontan procedure for pulmonary atresia and right ventricle hypoplasia. We focused on maintaining enough blood volume for cardiac output without causing pulmonary edema, as the patient is not well adapted to changes in volume. Owing to a multidisciplinary approach, the surgery was successfully performed without fatal adverse events.
Liver disease is a late complication of the Fontan procedure, a treatment used in cyanotic congenital heart disease. The Fontan procedure is a complex cardio-surgical process used in patients with single ventricular hearts that diverts systemic venous blood directly into the pulmonary arteries, bypassing the right ventricle [1]. In previous studies, the overall 1-, 5-, and 10-year survival rates of patients who underwent the Fontan procedure were found to be 77%, 70%, and 60%, respectively [2,3]. However, with recent improvements in surgery and anesthesia, the procedure has increased the long-term survival rate of patients, with 83% surviving to 20 years without the need for cardiac transplantation [2]. However, this increased life expectancy has led to not only late cardiac but also extra-cardiac complications. Chronic complex hemodynamic changes affect a wide range of organ systems, resulting in peripheral venous insufficiency, protein-losing enteropathy, and Fontan-associated liver disease (FALD), the latter of which includes liver cirrhosis (LC) and hepatocellular carcinoma (HCC) [4].
The etiology of FALD is a complex one, including both chronic congestive venous overflow and systemic hypoxia secondary to left ventricular dysfunction and diffuse pulmonary veno-venous shunts [5]. In patients with end-stage liver disease, the use of liver transplantation in the treatment of both HCC and LC has been well established. However, the Fontan circulation, characterized by elevated central venous pressure (CVP) and reduced cardiac output, is a challenging issue for both transplant surgeons and anesthesiologists. Moreover, as patients treated with the Fontan procedure represent a growing population that can survive well into adulthood, and the incidence of both LC and HCC increases with the duration of the Fontan circulation, the number of patients who need liver transplantation due to FALD will most likely continue to increase [1,2]. Therefore, it is important to understand the physiology of patients with FALD to determine the appropriate intraoperative management.
The first case series to discuss the development of HCC after the Fontan procedure was published in 2013. To the best of our knowledge, only 18 cases have been reported in the literature, with just a few patients for whom curative treatment with modalities such as hepatectomy was suitable [1]. Herein, we report a case of successful isolated living-donor liver transplantation for the treatment of HCC in a 24-year-old male patient who survived after a Fontan procedure for pulmonary atresia and severe right ventricular hypoplasia. Written informed consent was obtained from the patient for publication of this case report.
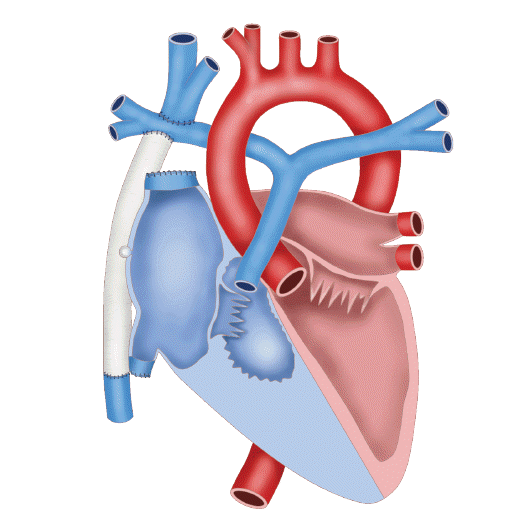
The patient was prenatally diagnosed with both pulmonary atresia with an intact ventricular septum and severe right ventricular hypoplasia. He showed cyanosis due to congenital heart disease immediately after birth. At the time of birth, his oxygen saturation level was 75–80%, requiring oxygen supply. Prostaglandin was continuously administered for patent ductus arteriosus. On the fifth day after birth, right-modified Blalock-Taussig shunt and right ventricular outflow tract widening were performed simultaneously to enhance pulmonary flow. When he was 6 months old, bidirectional cavo-pulmonary shunt and right pulmonary artery angioplasty were performed as a bridging therapy for congenital heart disease. One year after his most recent surgery, he underwent a Fontan operation with adjustable fenestration (Fig. 1). After the Fontan procedure, his oxygen saturation level improved to 90–95% without oxygen supply, and he lived without inconvenience in his daily life for 17 years. However, when he was 18 years old, dyspnea developed during exercise. Significant narrowing and diffuse hypoplasia were identified in the left pulmonary artery, for which a stent was inserted. During the workup, liver magnetic resonance imaging showed multiple HCCs in S3 and S7. Transcatheter arterial chemoembolization and radiation therapy for HCC were performed for 1 month with the expectation of cancer down-staging. For curative therapy, he was placed on the waiting list for liver transplantation.
When he was 24 years old, living-donor liver transplantation was planned. During the preoperative workup, his oxygen saturation was 80–85% on room air; however, after administering 3 L/min of oxygen via nasal cannula, oxygen saturation was maintained at 87–92%. A laboratory evaluation revealed the following results: a hemoglobin concentration of 20.2 g/dl, platelet count of 133 K/µl, creatinine level of 0.7 mg/dl, bilirubin level of 3.2 mg/dl, international normalized ratio level of 1.12, and sodium concentration of 139 mmol/L. Left ventricle (LV) cavity size, LV function, and conduit function were all normal upon preoperative examination using echocardiography. Flow in the left pulmonary arterial stent was sluggish, and fenestration flow was patent, with a peak velocity of 1.0 m/sec in the former. Since the baffle between the inferior vena cava and the pulmonary artery had a small leakage, a right-to-left shunt was observed in the patient. The ejection fraction was 74%, and regional wall motion abnormality was not observed.
The donor was a 55-year-old female (weight, 75.6 kg; height, 173 cm; body mass index, 25 kg/m2) and the patient’s mother. She was not found to have any comorbidities. The height and weight of the recipient were 158.7 cm and 49.6 kg, respectively. Computed tomography volumetric analysis of the donor’s liver showed an adequate right liver volume, and the calculated graft-to-recipient weight ratio was 0.87. She was deemed to be a suitable single donor. Preoperatively, an 11.5 Fr I-J catheter was inserted into the right internal jugular vein by a radiologist. The distal tip of the catheter was positioned on the superior vena cava. An air filter was applied to his intravenous line to prevent the entrance of air via the right-left shunt. Before induction, nasogastric tube insertion was attempted for esophageal varix; however, the patient did not cooperate owing to discomfort. His oxygen saturation before induction was 88% on room air. After pre-oxygenation with 100% oxygen via a facial mask, general anesthesia was induced with etomidate (14 mg), midazolam (2 mg), and rocuronium (80 mg). Before the application of the surgical retractor, anesthesia was maintained with sevoflurane and 50–100% oxygen. A 7 Fr 3-lumen central venous catheter was inserted into the right basilic vein under ultrasound guidance. The right radial and femoral arteries and right femoral vein were cannulated for continuous monitoring and frequent sampling.
After induction, nasogastric tube insertion was attempted but failed again because of bleeding. Transesophageal echocardiography (TEE) was originally planned; however, this decision was revised as TEE insertion is contraindicated in patients with esophageal varix. Anesthesia was maintained using isoflurane, remifentanil, and cisatracurium. The initial CVP was 15 mmHg and was maintained between 10 and 25 mmHg. CVP was monitored via a central venous catheter inserted into the internal jugular vein. The EV1000 clinical platform (Edwards Lifesciences, USA) was used to monitor hemodynamic parameters, such as CO, SV, systemic vascular resistance, and stroke volume variation (SVV). Pulse pressure variation (PPV) was monitored via femoral arterial cannulation. CVP, SVV, and PPV were used to ensure an adequate volume status. Cardiac output was maintained between 6 and 10 L/min, and oxygen saturation was maintained between 88% and 98% (Table 1). The target SpO2 was above 90% at a FiO2 of 0.5. Isoflurane was maintained between 1 and 1.5 MAC.
After his liver was explanted, a porto-caval shunt was performed. The mean hepatic duration was 125 min. After portal vein anastomosis, the graft was reperfused by consecutively unclamping the hepatic and portal veins. Hypotension was observed in the patient, whose mean arterial blood pressure was 50 mmHg. As treatment for hypotension, 0.1 ㎍/kg/min of norepinephrine was continuously infused. Hepatic artery anastomosis was performed after reperfusion, after which biliary anastomosis was performed and a porto-caval shunt was ligated. The patient was transferred to the surgical intensive care unit (ICU) and administered 0.1 ㎍/kg/min of norepinephrine. The transfer was uneventful.
The total operating time was 516 min. The cold and warm ischemic times were 141 and 64 min, respectively, and there were few ascites. The estimated blood loss (expressed as lost red blood cell mass) was 450 ml, and 300 ml of salvaged blood was transfused without allogeneic red blood cells [6]. During the operation, 7,610 ml of crystalloid without colloid was infused. The total urine output was 910 ml.
On the day of the operation, the patient was returned to the operating room for bleeding control. Active bleeding from the hepatic artery anastomosis site was controlled, and he was discharged to the ICU again. The patient was extubated on postoperative day (POD) 1. His vital signs were stable, and his liver function had improved. His heart function was stable during the regular checks with transthoracic echocardiography (ejection fraction 49.1–58.5%). On POD 5, he was transferred to the general ward. In the ward, 3 L/h of oxygen was applied via the nasal cannula, and his oxygen saturation was maintained at 88–90%. On POD 10, he underwent reoperation for bleeding control, but surgeons could not find a specific focus of bleeding. The mid-portion of the wound was maintained in an open state, and gauze packing was applied while a penrose drain was inserted. The wound site exuded for 18 days after the surgery, and the patient was discharged on POD 27 because of delayed wound repair.
Fontan circulation affects the dual blood supply of the liver and is responsible for the injury pattern commonly seen in FALD. In the Fontan circulation, the hepatic veins drain directly into the Fontan circuit, and the liver is therefore particularly susceptible to the effects of central venous hypertension [3]. Cirrhosis is the single most important risk factor for the development of HCC [7], which has been recognized as an uncommon complication of FALD in some reports. Cirrhosis may develop approximately 11–15 years after the Fontan procedure, and there are both case series and reports of HCC having complicated cirrhosis in FALD patients [8–11]. However, there is limited evidence regarding the optimal treatment strategy for HCC complicated by FALD. Liver transplantation might be considered in patients with early stage HCC; however, the circulatory characteristics of the Fontan circulation limits the use of this method. Hence, previous studies have recommended heart-liver transplantation for these patients. Evidence of the effectiveness or safety of isolated liver transplantation in adult patients with severe congenital heart disease is limited. Conversely, liver transplantation has been successfully performed in children with congenital heart disease, and a successful case of pediatric liver transplantation has been reported following a Fontan procedure in left isomerism combined with biliary atresia [12]. Most adult patients with failed Fontan procedures have significantly elevated right atrial pressure. In some hospitals, isolated liver transplantation is considered a relative contraindication when patients have right atrial pressure greater than 15 mmHg [5]. A high CVP may also increase bleeding risk during surgery, and a decrease in systemic vascular resistance after reperfusion may lead to further intra-cardiac shunting (right to left) and hypoxia [13]. However, in the present case, the Fontan circulation was relatively well preserved, right atrial pressure was 14–17 mmHg, and the ejection fraction was 74%; therefore, isolated liver transplantation was decided upon following a multidisciplinary discussion.
In patients with Fontan circulation, the most challenging issue during liver transplantation is maintaining sufficient blood volume for cardiac output without causing pulmonary edema. This is owing to the documented cases of patients not being able to adapt to volume changes [5]. To achieve a successful outcome, there are several important principles to consider. First, from an anesthesiologist’s perspective, it is of paramount importance that cardiac contractility and a high CVP be preserved. However, it is challenging to maintain such a state because a high CVP may lead to excessive bleeding during surgery. Hence, volume management with crystalloid, colloid, and blood products should be adjusted carefully. The use of an inodilator may be an option for patients with Fontan circulation if the CVP or arterial blood pressure has not been adequately maintained during surgery, especially after reperfusion. One such inodilator, Milrinone, is a phosphodiesterase type III inhibitor that works to increase the heart's contractility and decrease pulmonary vascular resistance. Second, the potential for air embolism, which can lead to either pulmonary embolism or paradoxical emboli and cerebral infarction, needs to be considered [14,15]. Moreover, in patients with Fontan circulation, the risk of thromboembolism is higher because the pulmonary blood flow remains non-pulsatile as venous flow takes place without the right ventricle. Therefore, the use of an air filter and close observation with TEE can be useful. However, because patients who undergo the Fontan procedure usually have severe esophageal varix, the decision to use TEE should be preceded by a thorough risk-benefit assessment. Third, from the surgeon’s standpoint, meticulous surgery to reduce blood loss is mandatory. At our institution, we attempted to minimally clamp the inferior vena cava during the anhepatic phase while also monitoring femoral vein pressure, the latter of which represents inferior vena cava pressure and helps to maintain CVP. The creation of a transient porto-caval shunt can be an effective method to reduce temporal portal hypertension during the anhepatic phase and increase central venous volume.
In our case, the patient suffered wound problems and underwent additional operations for bleeding control after the liver transplantation, which led to a prolonged hospital stay. Delayed wound healing could potentially be the result of hypoxia caused by the Fontan circulation.
In conclusion, a living-donor liver transplant was used to successfully treat HCC in a 24-year-old male patient who survived a Fontan procedure for pulmonary atresia and severe right ventricular hypoplasia. To the best of our knowledge, this is the first case of isolated liver transplantation where the recipient became an adult after the Fontan procedure. In addition to a comprehensive understanding of the physiology of patients with FALD, a multidisciplinary approach should be used to assess successful outcomes in these patients.
REFERENCES
1. Mazzarelli C, Cannon MD, Hudson M, Heaton N, Sarker D, Kane P, et al. Hepatocellular carcinoma as a complication of vascular disease of the liver after Fontan procedure. Hepatology. 2019; 69:911–3.
2. Téllez L, Rodríguez-Santiago E, Albillos A. Fontan-associated liver disease: a review. Ann Hepatol. 2018; 17:192–204.
3. Gordon-Walker TT, Bove K, Veldtman G. Fontan-associated liver disease: a review. J Cardiol. 2019; 74:223–32.
4. Téllez L, Rodríguez de Santiago E, Minguez B, Payance A, Clemente A, Baiges A, et al. VALDIG an EASL consortium. Prevalence, features and predictive factors of liver nodules in Fontan surgery patients: the VALDIG Fonliver prospective cohort. J Hepatol. 2020; 72:702–10.
5. Asrani SK, Asrani NS, Freese DK, Phillips SD, Warnes CA, Heimbach J, et al. Congenital heart disease and the liver. Hepatology. 2012; 56:1160–9.
6. Bang SR, Ahn HJ, Kim GS, Yang M, Gwak MS, Ko JS, et al. Predictors of high intraoperative blood loss derived by simple and objective method in adult living donor liver transplantation. Transplant Proc. 2010; 42:4148–50.
7. European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–43.
8. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. 2005; 129:1348–52.
9. Asrani SK, Warnes CA, Kamath PS. Hepatocellular carcinoma after the Fontan procedure. N Engl J Med. 2013; 368:1756–7.
10. Saliba T, Dorkhom S, O'Reilly EM, Ludwig E, Gansukh B, Abou-Alfa GK. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol. 2010; 22:889–91.
11. Elder RW, Parekh S, Book WM. More on hepatocellular carcinoma after the Fontan procedure. N Engl J Med. 2013; 369:490.
12. Youn JK, Lee JM, Yi NJ, Choi YR, Suh SW, You T, et al. Pediatric split liver transplantation after Fontan procedure in left isomerism combined with biliary atresia: a case report. Pediatr Transplant. 2014; 18:E274–9.
13. Cetta F, Feldt RH, O'Leary PW, Mair DD, Warnes CA, Driscoll DJ, et al. Improved early morbidity and mortality after Fontan operation: the Mayo Clinic experience, 1987 to 1992. J Am Coll Cardiol. 1996; 28:480–6.
14. Manzoni D, D'Ercole C, Spotti A, Carrara B, Sonzogni V. Congenital heart disease and pediatric liver transplantation: complications and outcome. Pediatr Transplant. 2007; 11:876–81.
15. Garbanzo JP, Kasahara M, Egawa H, Ikeda T, Doi H, Sakamoto S, et al. Results of living donor liver transplantation in five children with congenital cardiac malformations requiring cardiac surgery. Pediatr Transplant. 2006; 10:923–7.
Table 1.
Hemodynamic Changes & Oxygenation Status During Liver Transplantation




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download