INTRODUCTION
MATERIALS AND METHODS
1) Magnesium sulfate group (Group M, n = 33): 40 ml solution of 40 mg/kg of magnesium sulfate and isotonic saline is injected at a rate of 120 ml/h for 20 min as a bolus immediately prior to anesthesia induction. Subsequently, 40 ml of the same solution is infused at a rate of 10 ml/h by continuous intravenous infusion until the end of the operation (for an infusion of 10 mg/kg/h).
2) Vitamin C group (Group V, n = 33): 40 ml of a solution of 50 mg/kg of vitamin C (Ascorbic Acid Injection®, Huons Co., Korea) and isotonic saline is injected at a rate of 120 ml/h for 20 min as a bolus immediately prior to anesthesia induction. Subsequently, 40 ml of isotonic saline is infused at a rate of 10 ml/h by continuous intravenous infusion until the end of the operation.
3) Magnesium sulfate and vitamin C group (Group MV, n = 33): 40 ml solution of 40 mg/kg of magnesium sulfate and 50 mg/kg of vitamin C with isotonic saline is injected at a rate of 120 ml/h for 20 min as a bolus immediately prior to anesthesia induction. Subsequently, 40 ml of a solution of 40 mg/kg of magnesium sulfate and isotonic saline is infused at a rate of 10 ml/h by continuous intravenous infusion until the end of the operation.
4) Control group (Group C, n = 33): 40 ml of isotonic saline is injected at a rate of 120 ml/h for 20 min as a bolus immediately prior to anesthesia induction. Subsequently, 40 ml of isotonic saline is infused at a rate of 10 ml/h by continuous intravenous infusion until the end of the operation.
RESULTS
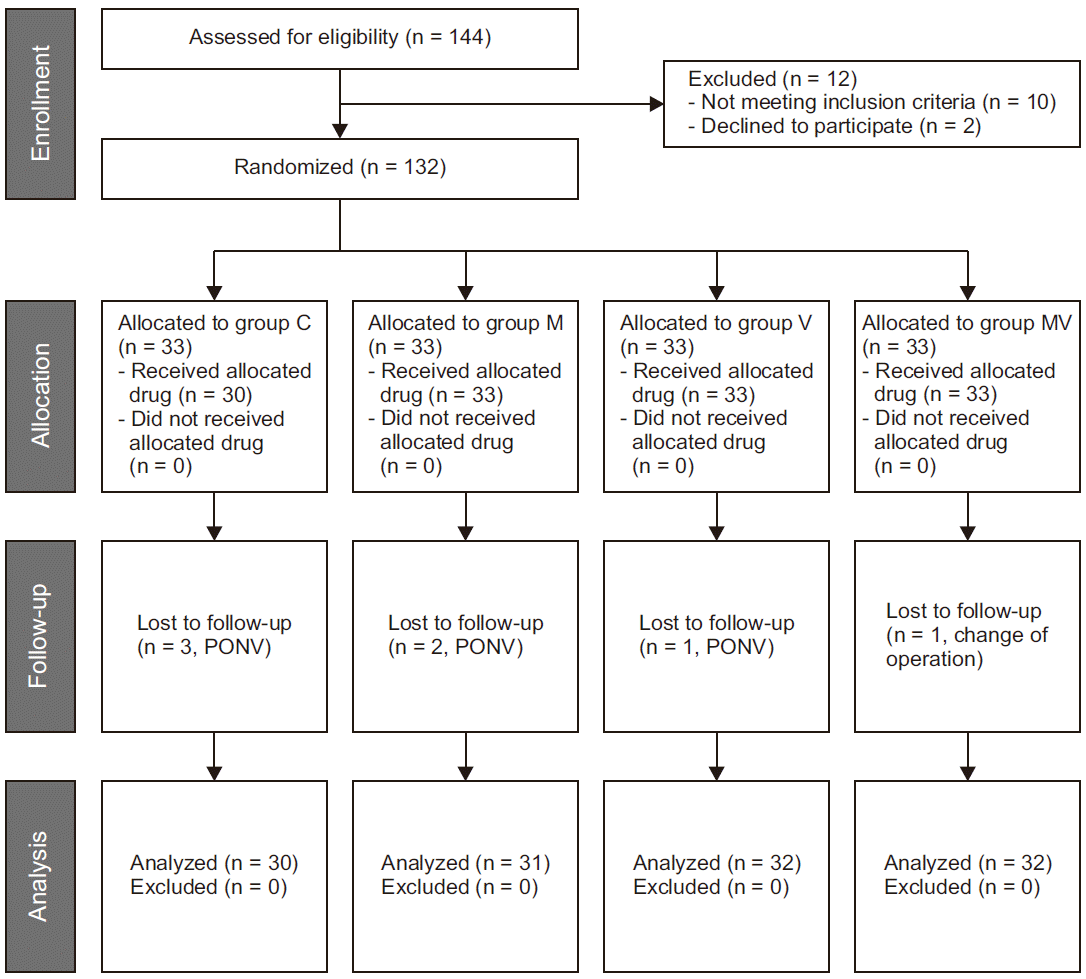 | Fig. 1CONSORT flow diagram. Group C: control group, Group M: magnesium sulfate group, Group V: vitamin C group, Group MV: magnesium sulfate + vitamin C group. PONV: postoperative nausea and vomiting. |
Table 1
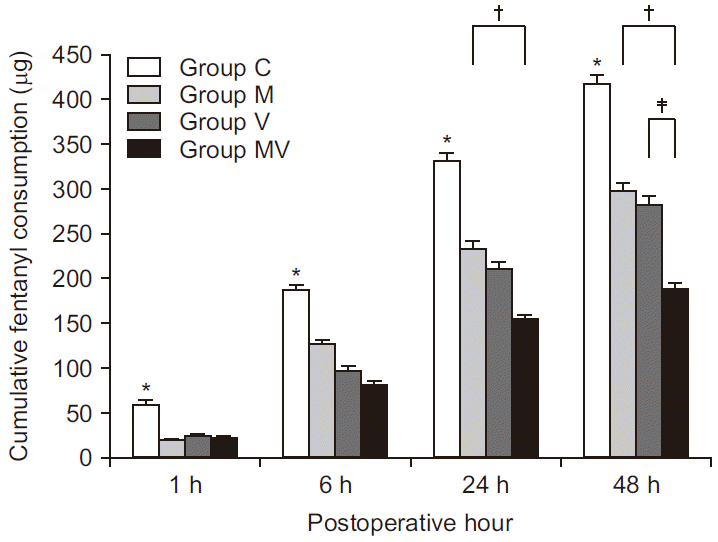 | Fig. 2Cumulative fentanyl consumption. Mean cumulative injected fentanyl dose of the intravenous patient-controlled analgesia solution in the four groups. The error bars show standard deviation. Group C: control group, Group M: magnesium sulfate group, Group V: vitamin C group, Group MV: magnesium sulfate + vitamin C group. *Indicates significant difference between Group C and the other groups (P < 0.008). †Indicates significant difference between Group MV and Group M (P = 0.001, P < 0.001, 24 and 48 h after operation, respectively). ‡Indicates significant difference between Group MV and Group V (P = 0.003). |
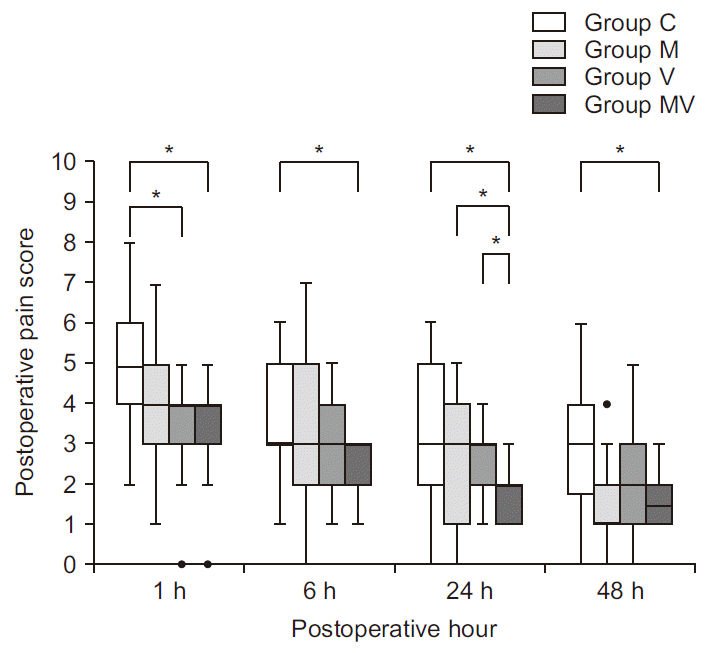 | Fig. 3Postoperative pain scores. Postoperative pain scores by numeric rating scale at 1, 6, 24, 48 h after surgery. Box plot with median (solid line), interquartile ranges (box) and values within 1.5 interquartile ranges from each side of the box (whiskers). Outliers are indicated by solid circles. Group C: control group, Group M: magnesium group, Group V: vitamin C group, Group MV: magnesium + vitamin C group. *Indicates significant difference between groups (P < 0.008). |
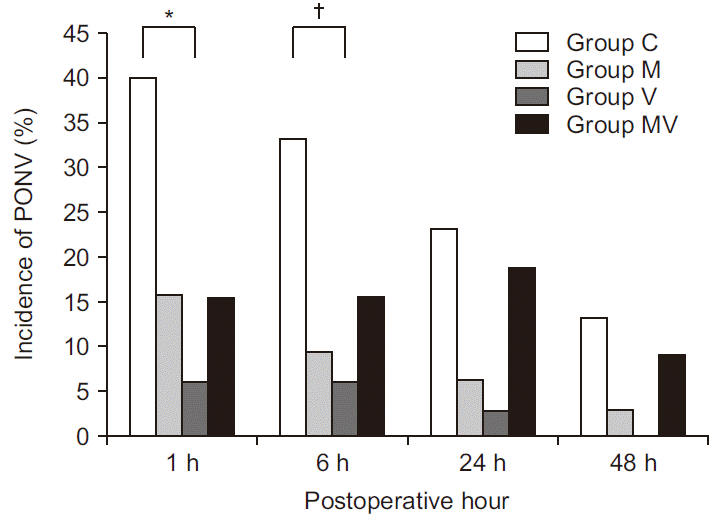 | Fig. 4Incidence of postoperative nausea and vomiting. Values are presented as number (%). Group C: control group, Group M: magnesium group, Group V: vitamin C group, Group MV: magnesium + vitamin C group, PONV: postoperative nausea and vomiting. *Indicates significant difference between Group C and Group V (P = 0.001). †Indicates significant difference between Group C and Group V (P = 0.007). |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download