An 18-year-old man (175 cm, 81 kg) with right hemifacial microsomia was scheduled for double-jaw surgery, correction with distraction osteogenesis, and mandibular anglectomy. Although he had no underlying medical history, he had undergone correction with distraction osteogenesis in 2006. At that time, the patient was classified as Cormack-Lehane grade 4, and oral intubation was performed with FOB. The patient reported a history of sleep apnea and heavy snoring, during the preoperative interview. His mouth opening was about 2.5-finger-breadth and neck extension was normal, during the preoperative physical examination. However, he had severe retrognathia and a class III Mallampati score. The thyromental distance was less than 6 cm. Moreover, an oral panoramic view X-ray showed narrowing of the nasopharyngeal airway, due to severe retrognathia (
Fig. 1). According to El-Anwar et al. [
9], the mean depth (anteroposterior diameter) of the nasopharynx in normal adults is 21.8 ± 4.6 mm. However, the depth of nasopharynx in our patient was 15.5 mm, which was narrower than usual. We explained the possibility of a difficult airway and informed the patient about the special risks and procedures pertaining to performing an awake intubation procedure. Other preoperative laboratory examinations, chest X-ray, and electrocardiography revealed no abnormalities.
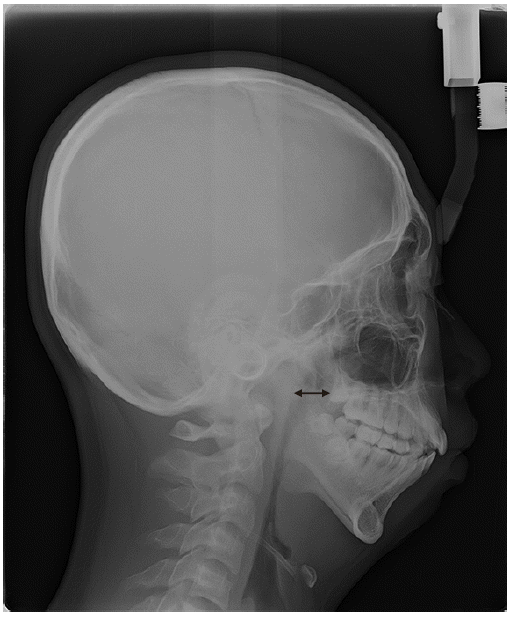 | Fig. 1Narrowing of the nasopharyngeal airway seen on the oral panoramic view X-ray (double-headed arrow). 
|
On the day of surgery, we prepared a Glidescope
®; LMA, fiberoptic bronchoscope (Olympus Optical, Japan); a 0.035-inch-thick, 145-cm-long straight-tipped Angio Guidewire (Lunderquist-ring, Cook Critical Care,
Fig. 2); and a high-flow nasal cannula (Optiflow™). Nasotracheal intubation was required to secure space in the oral cavity for surgery; thus, we prepared nasotracheal tubes of various sizes. Non-invasive blood pressure monitoring, electrocardiogram, pulse oximetry, and bispectral index (BIS) measurement were performed in the operating room. The monitor showed an initial blood pressure of 140/84 mmHg, a heart rate of 58 beats/min and room air saturation of 98%. Lidocaine 4% was sprayed onto the tongue and oropharynx for topical anesthesia. Sufficient preoxygenation was provided with Optiflow™, at a flow rate of 20 L/min, to achieve an SpO
2 of 100%. We started total intravenous anesthesia (TIVA) with 2% propofol and remifentanil administered at effect-site concentrations of 2.5 µg/ml and 1.5 ng/ml, respectively, with a target-controlled infusion pump. The SpO
2 decreased to 96%, soon after the BIS was 42 and the patient fell asleep, but there was no further desaturation, after we raised the flow rate of Optiflow™ to 40 L/min. Since the left nostril was larger than the right nostril, owing to right-sided hemifacial microsomia, we decided to insert the FOB into the left nostril. After guiding the FOB into the left nostril of the patient, it passed into the trachea through the glottis, without any issue. We inserted the 145-cm-long, straight-tipped Angio Guidewire (Lunderquist-ring, Cook Critical Care) into the working channel of the FOB, till we could visualize the entrance of the guidewire into the carina (
Fig. 3). We carefully removed the FOB and the guidewire remained in position. We also used the Glidescope
® to confirm that the guidewire was still within the oral cavity and had passed through the vocal cord, and into the trachea (
Fig. 4). The exchange catheter (1.6-mm internal diameter [ID], 2.7-mm outer diameter, CAEC, Cook Critical Care) was inserted over the Angio Guidewire and the Angio Guidewire was pulled out. A nasotracheal tube with 6.5 mm ID was advanced carefully over the exchange catheter into the airway without incidence. After removing the exchange catheter, the tracheal tube cuff was inflated, and proper positioning of the ETT was confirmed by auscultating both lungs and by confirming continuous positive end-tidal CO
2. The monitor showed a blood pressure of 133/86 mmHg, heart rate of 95 beats/min, an SpO
2 of 97%, and a BIS of 50 after intubation. An oxygenation level of 97% or higher was maintained throughout the procedure. After confirming proper intubation, we adjusted the effect-site concentration of propofol and remifentanil to 4.0 µg/ml and 2.5 µg/ml, respectively, and administered 80 mg of rocuronium intravenously. The time required for the administration of intubation to its confirmation was 16 min. The intra and postoperative periods were uneventful.
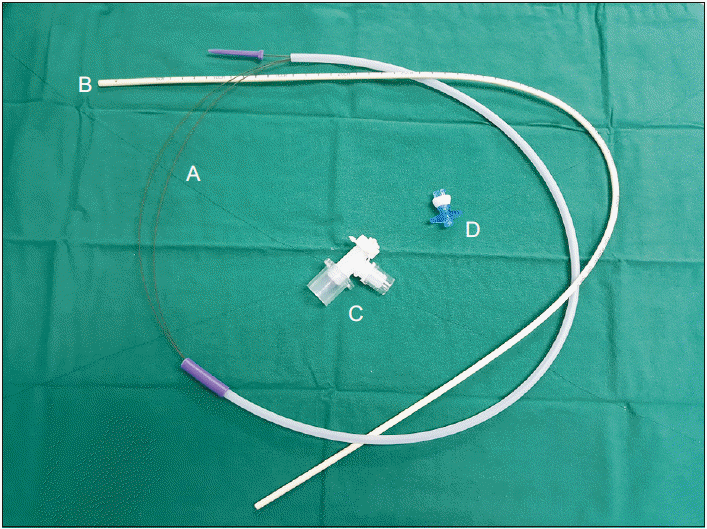 | Fig. 2Angio Guidewire and airway exchange catheter set A: straighttipped Angio Guidewire (Lunderquist-ring, Cook Critical Care), B: exchange catheter (CAEC, Cook Critical Care), C: bronchoscope airway adapter, D: Rapi-Fit® adapter. 
|
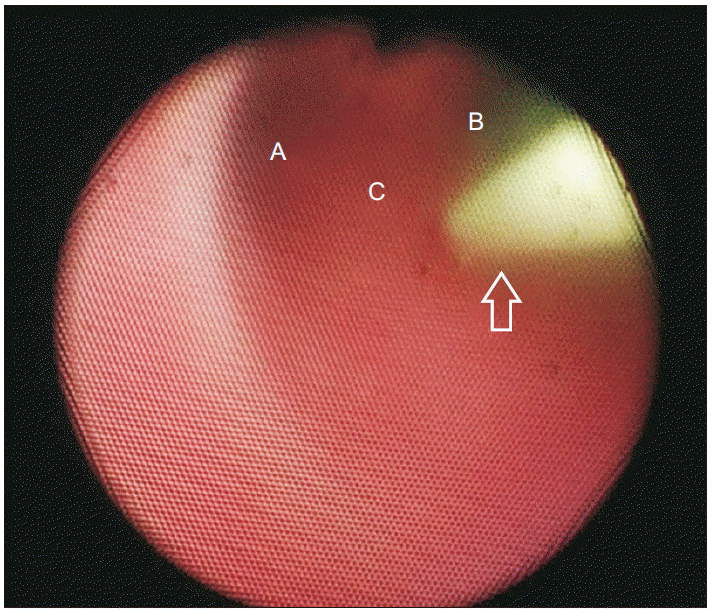 | Fig. 3The fiberoptic bronchoscope showing that the guidewire is positioned in the trachea A: left main bronchus, B: right main bronchus, C: carina, Empty arrow: guide wire. 
|
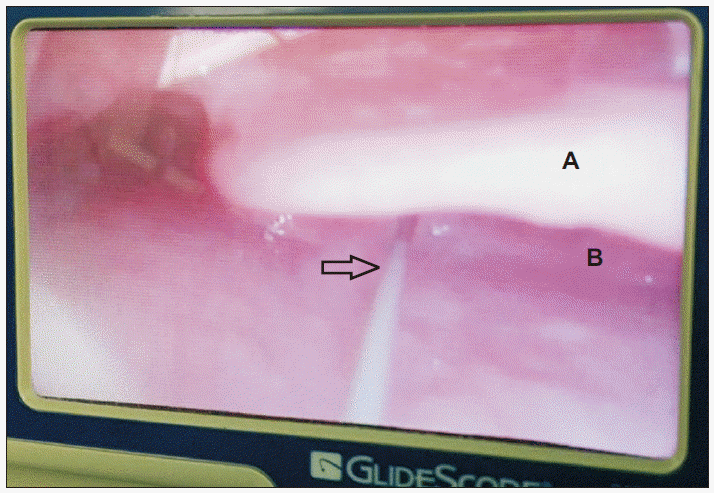 | Fig. 4The guidewire passing through the vocal cord and into the trachea as seen with the Glidescope® A: epiglottis, B: arytenoid cartilage, Empty arrow: guide wire. 
|
After the procedure, we provided a full explanation regarding this case report to the patient and obtained permission for the same.

