INTRODUCTION
Sepsis, an uncontrolled host response to infection, may be life-threatening organ dysfunction [
1]. Despite the use of novel modern antibiotics and therapeutic interventions, sepsis remains a common worldwide health issue due to increase in ageing populations and high death rates. The mortality rate resulting from adult sepsis remains as high as 21–35% [
2–
4].
Neutrophils play a critical role in combating infections and control of the immune response. Under non-infectious conditions, neutrophils are functionally quiescent with short lifespans (7–12 h in vivo), and the number of neutrophils is kept constant through apoptosis. When neutrophils become activated under infectious conditions, the functionality of neutrophils markedly increase with prolonged survival. This process is advantageous in most infectious conditions [
5]. However, over-activated neutrophils can result in massive and uncontrolled secretion of pro-inflammatory cytokines including tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-8 and activation of mitogen-activated protein kinase (MAPK) pathway such as p38, extracellular-signal-regulated kinase (ERK)1/2, and c-Jun N-terminal kinase (JNK). These processes may cause host tissue injury in excessive inflammatory condition [
6–
8]. In particular, TNF-α, IL-6, and IL-8 are essential for the eradication of pathogens. However, the over-expression of inflammatory cytokines provokes life-threatening conditions, including sepsis and septic shock [
8]. Therefore, the inhibition of pro-inflammatory cytokines, prevention of MAPKs activation, and modulation of neutrophil apoptosis should be important strategies for the treatment of excessive inflammatory diseases, such as sepsis and septic shock.
Nontoxic substances that regulate the production-mediated inflammatory responses of neutrophils may be a novel therapy in sepsis treatment; thus, many studies have attempted to identify candidate substances. Curcumin (1,7-bis-[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione) is a yellow pigment of turmeric that is derived from the rhizome of the
Curcuma longa plant and is widely used as a spice and food coloring agent. Curcumin can improve the condition in sepsis due to its anti-inflammatory, antioxidant and immunomodulatory properties. Curcumin inhibits the secretion of plasma TNF-α and IL-6, the activation of MAPKs, and the production of reactive oxygen species (ROS) which relate to inflammatory response [
9,
10]. Curcumin acts on MAPK p38 pathway modulating COX-2 and iNOS expression in chronic experimental colitis [
11]. Curcumin also attenuates sepsis-induced acute organ dysfunction by preventing inflammation and enhancing the suppressive function of regulatory T cells [
12]. Despite the promising evidence of the therapeutic effects of curcumin on the sepsis complication, the role of curcumin on its effect and possible mechanisms of action in human studies is not well known, in particular, the effect on human neutrophil is not documented.
The primary aim of the present study was to evaluate the roles of curcumin on neutrophil apoptosis and activation that are associated with the levels of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-8, and the expression of intracellular signaling pathways, such as the MAPK pathway.
Go to :

MATERIALS AND METHODS
Isolation of neutrophils
The peripheral blood of healthy volunteers was used to isolate neutrophils under a protocol approved by the Chonnam National University Hospital Institutional Review Board (no. CNUH-2012-048). Dextran (6%) was added and erythrocytes were sedimented under gravity for 45 min at room temperature. Then leukocyte-enriched pellets were collected by centrifugation at 1,100 rpm for 6 min and re-suspended in platelet-poor plasma. Next, leukocyte-enriched plasma was centrifuged with a plasma-Percoll gradient (3 ml 42–51%) at 1,100 rpm for 10 min. Neutrophils were found at the 42–51% Percoll layer interface. Red blood cells (RBC) were removed by RBC Lysis Buffer and neutrophils were harvested by centrifugation at 3,000 rpm for 5 min. Finally, neutrophils were re-suspended in RPMI 1640 with 10% FBS and 1% streptomycin and penicillin (Mediatech, USA). The neutrophil viability as measured by the trypan blue exclusion test was more than 98% consistently.
Cytokine analyses
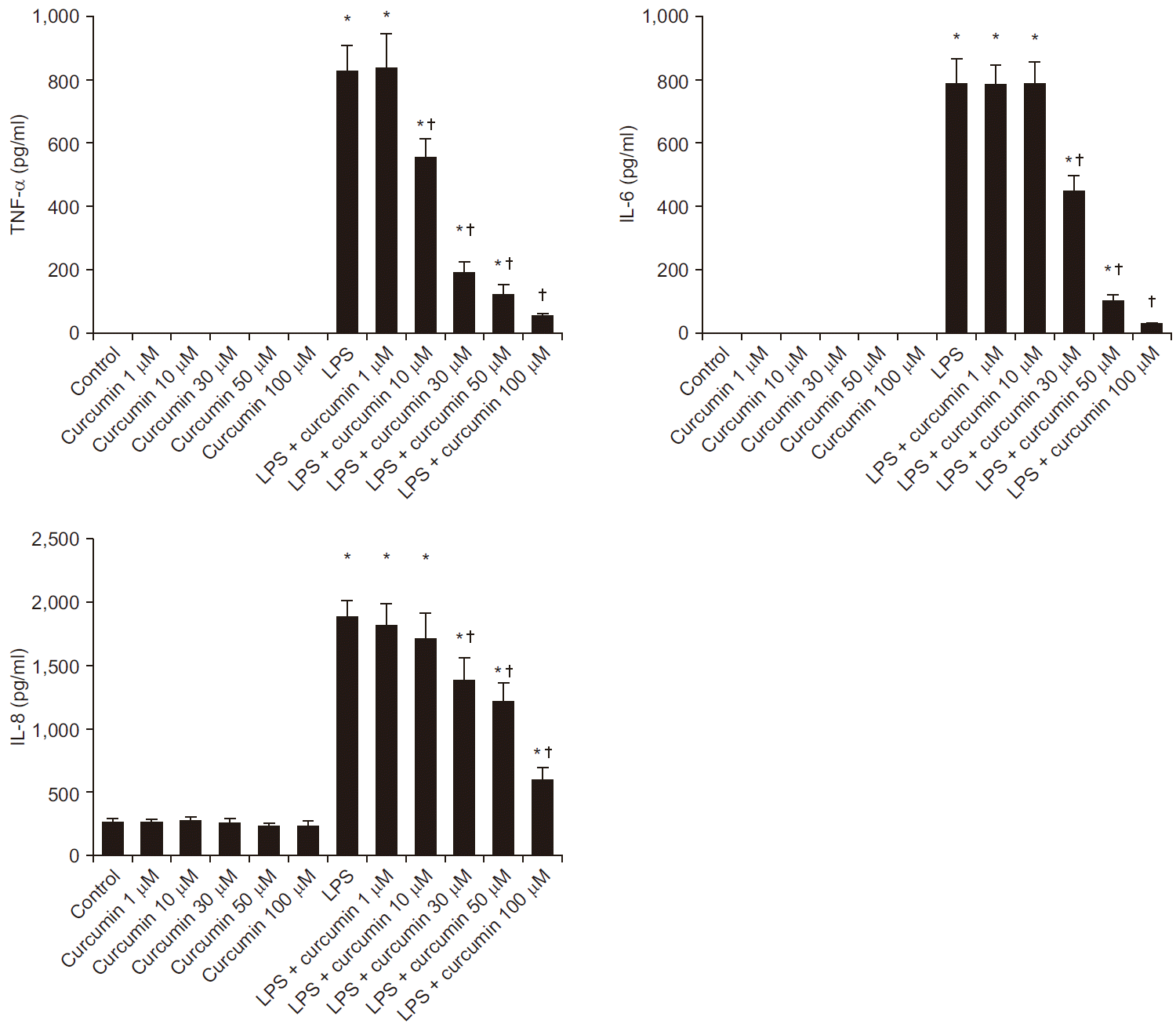
To measure the effects of curcumin on the levels of pro-inflammatory cytokines (TNF-α, IL-6, and IL-8) of lipopolysaccharide (LPS)-stimulated neutrophils, isolated human neutrophils (5 × 106/ml) were incubated without or with LPS (100 ng/ml, 055:B5 Escherichia coli endotoxin, Sigma-Aldrich, USA) and curcumin (0, 1, 10, 30, 50, 100 µM) in 24-well plates within 4 h. Pro-inflammatory cytokines (TNF-α, IL-6, and IL-8) were measured by enzyme-linked immunosorbent assay kits (R & D Systems, USA) according to the manufacturer’s instructions.
Western blot analysis
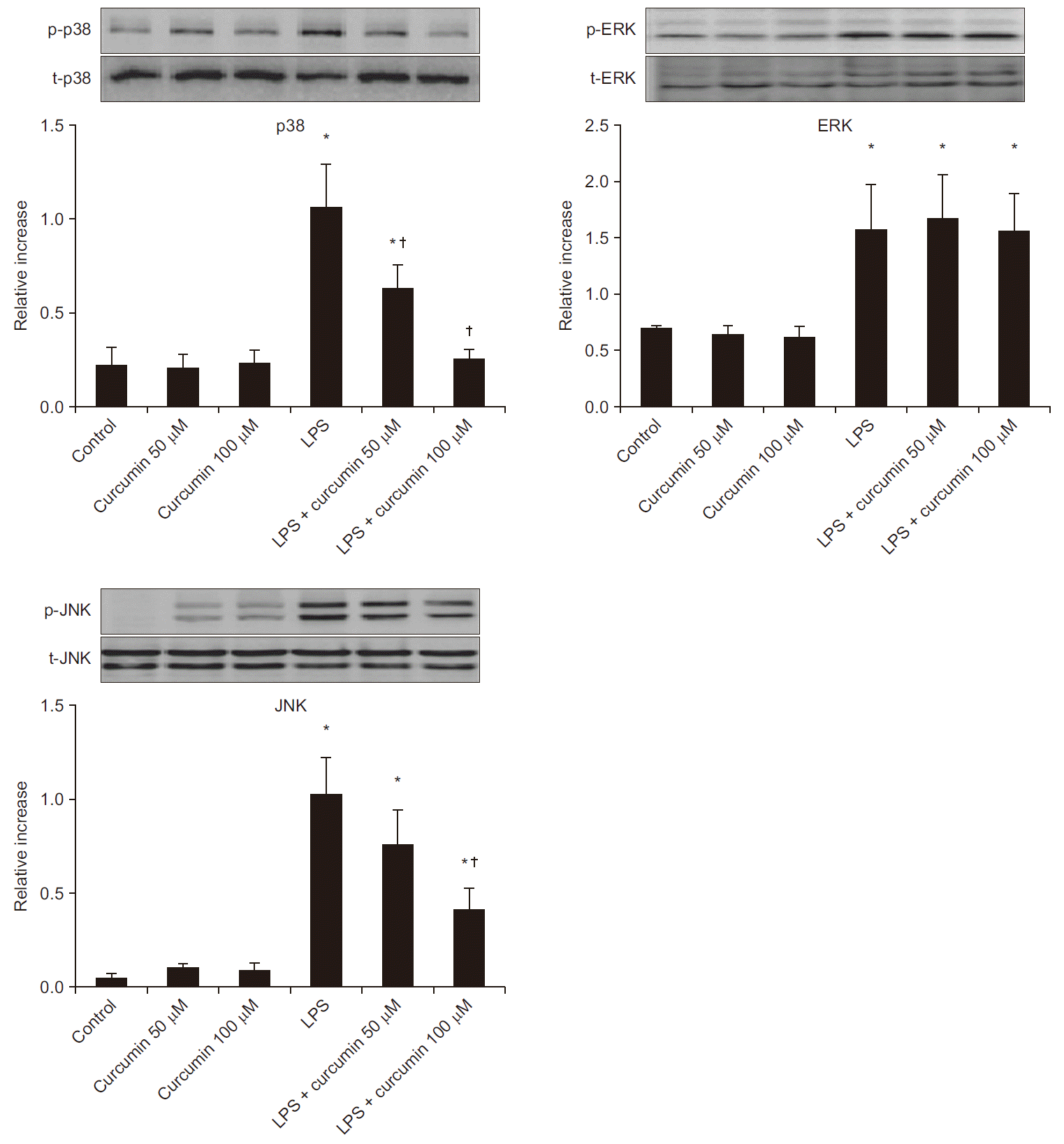
Phosphorylation of MAPKs was determined as follows: neutrophils were lysed in protein extraction solution (PRO-PREPTM, Intron biotechnology, Korea) after cultured with or without LPS (100 ng/ml) and curcumin (0, 50, 100 µM), incubated 30 min and then sonicated for 30 s. Debris from the lysed cell was pelleted by centrifugation at 14,000 for 20 min and supernatant was transferred into new tube and store –80°C until use. The protein concentration of each sample was assayed by the BCA kit (Pierce, USA), according to manufacturer’s protocol.
The levels of phosphorylated and total p38 MAPK, JNK, and ERK1/2 (Cell Signaling Technologies, USA) were detected by Western blot analysis. Next, we used a chemiluminescence system and analysis software (Bio-Rad, USA) to analyse the ratio between phosphorylated and total kinase.
Determination of neutrophil apoptosis by flow cytometry
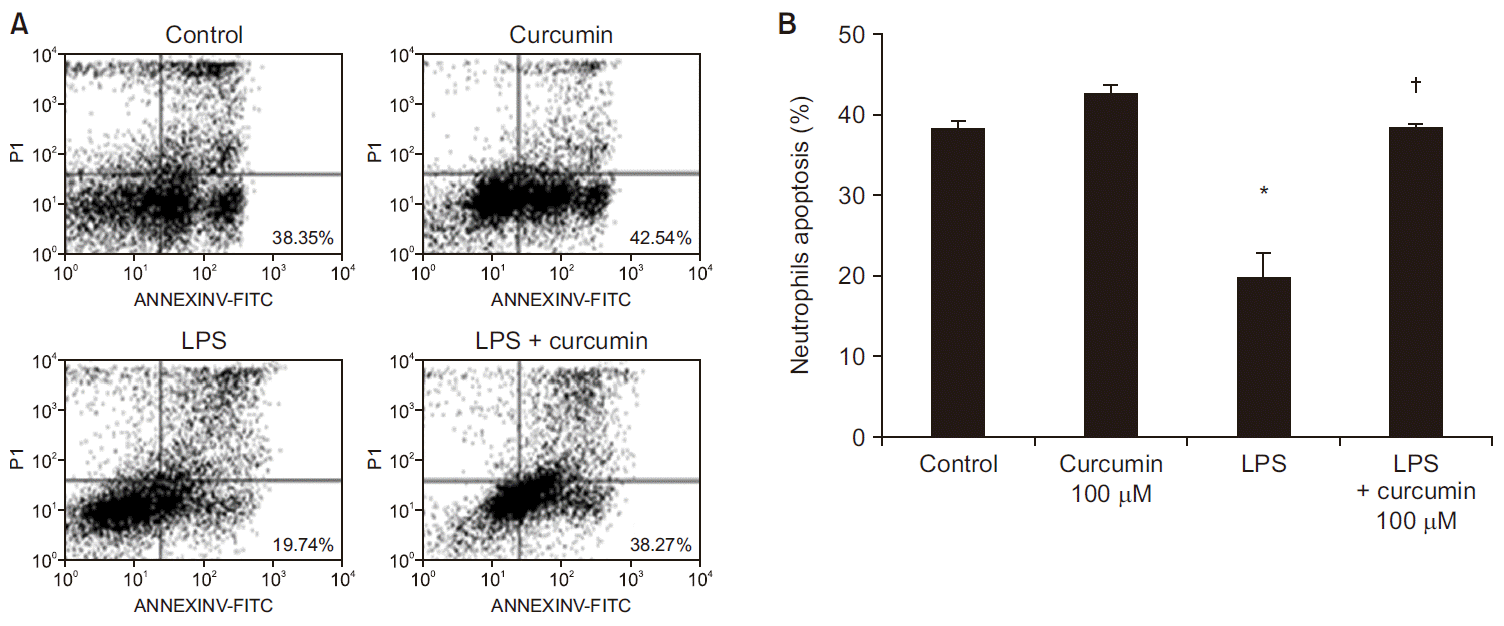
Neutrophil apoptosis was evaluated with fluorescein isothiocyanate annexin V/propidium iodide (FITC-Annexin V/PI) according to the manufacturer’s instructions (BD Biosciences, USA) with minor changes. To assess the effects of curcumin on neutrophil apoptosis, neutrophils were exposed without or with LPS (100 ng/ml) and curcumin (100 µM) for 24 h. They were washed with phosphate-buffered saline and centrifuged twice at 3,000 rpm for 5 min, after which the cells were incubated with 300 µl of binding buffer containing annexin V and PI for 15–20 min, followed by flow cytometry within 1 h after annexin V/PI labeling. Neutrophils in the process of apoptosis were determined by positive FITC-annexin V staining and negative PI staining.
Statistical analysis
Data are expressed as the mean ± standard deviation for each group and analyzed by one-way analysis of variance followed by the Tukey-Kramer multiple comparisons test or Student’s t-test using SPSS version 21 (IBM Co., USA). Statistical significance was defined as P < 0.05.
Go to :

DISCUSSION
The primary aim of the present study was to explore the effects of curcumin on LPS-induced neutrophil activation in terms of the stimulation of pro-inflammatory cytokines (TNF-α, IL-6, and IL-8), the phosphorylation of MAPKs (p38, ERK1/2, and JNK), and neutrophil apoptosis. The principal finding of the present study was that curcumin play a key role in anti-inflammatory activities, such as reducing the expression of MAPKs (p38 and JNK but not ERK1/2), decreasing the level of cytokines (TNF-α, IL-6, and IL-8), and enhancing neutrophil apoptosis by LPS-stimulated neutrophils under in vitro conditions.
LPS-induced neutrophils activate a variety of kinases associated with pro-inflammatory cytokine activation. Of these kinases, the signaling pathway induces the activation of p38, ERK1/2, and JNK which is particularly important because the decrease of MAPKs activation markedly controls the mediators of cytokine activation induced by LPS [
6,
7]. Under these signaling pathways, neutrophils release the numerous of oxygen radicals, proteases, leukotrienes, and pro-inflammatory cytokines [
13,
14]. Thus, inhibition of these signaling pathway may be a key strategy for the treatment of sepsis.
Inhibitors of nuclear factor kappa b (NF-κB), such as curcumin, may reduce cytokine activities. For example, pretreatment with curcumin attenuates hemorrhage/resuscitation injury-induced increases in pro-inflammatory cytokines and also inhibits the activation of NF-κB and protein-1 [
15]. Treatment with curcumin mitigates the systemic response to sepsis rats by cecal ligation and puncture. Furthermore, the intravenous administration of curcumin attenuates tissue injury, reduces death rate, and decreases the TNF-α protein levels [
16].
The present study assessed MAPKs activity using western blot analyses because p38, ERK1/2, and JNK, which are all MAPKs, are associated with cellular damage and/or cell death [
17,
18]. These compounds are stimulated by extracellular stressors such as ROS and cytokines and can cause cell death. Moreover, MAPKs are important upstream modulators of the production of pro-inflammatory mediators and enzymes [
19,
20], and LPS can upregulate NF-κB activity via MAPK signaling [
21]. In the present study, curcumin inhibited the phosphorylation levels of p38 and JNK, that were induced by LPS stimulation in neutrophils. Curcumin also likely inhibited the LPS-induced activation of p38 and JNK via alterations in NF-κB signaling. Taken together, the inhibition of pro-inflammatory activities by curcumin probably occurred via pathways that converge on p38 or JNK because these kinases regulate the production of TNF-α, IL-6, and IL-8 by LPS-stimulated neutrophils.
In this study, the rate of neutrophil apoptosis was decreased by LPS-stimulated neutrophils, whereas treatment with curcumin and LPS restored the decreased rate of neutrophil apoptosis in LPS-stimulated neutrophils. Under normal conditions, neutrophils have a short half-life (7–12 h in vivo) but exhibit an extended lifespan during sepsis. It has also been shown that several signaling pathways contribute to the resistance of neutrophils to apoptosis. For example, high levels of TNF-α, IL-1β, IL-6, IL-8, interferon gamma, granulocyte-colony stimulating factor, and granulocyte-macrophage colony-stimulating factor in conjunction with LPS stimulation delay neutrophil apoptosis. Additionally, a prolonged neutrophil lifespan contributes to bacteria killing. However, the persistent delay of neutrophil apoptosis is involved in acute inflammatory diseases and can induce tissue injuries through their secreted products [
5].
The present results are consistent with those of the above studies, because, under stimulation by LPS, they showed that neutrophils released large quantities of pro-inflammatory cytokines (TNF-α, IL-6, IL-8) that subsequently suppressed neutrophil apoptosis. Additionally, activation of the MAPKs signaling pathways was significantly accelerated in the presence of LPS. Therefore, the inhibition of inflammatory cytokines, prevention of MAPKs activation, and modulation of neutrophil apoptosis should be important strategies for the treatment of inflammatory diseases.
In summary, the present study showed that curcumin suppressed the production of TNF-α, IL-6, and IL-8 by inhibiting P38 and JNK activation pathway and enhancing apoptosis in LPS-stimulated human neutrophils. Our results reveal the underlying mechanism of how curcumin attenuate neutrophil activation and suggest potential clinic applications of curcumin supplementation for patients with severe sepsis and septic shock. However, additional clinical studies are required to confirm these in-vitro findings.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download