INTRODUCTION
Nicardipine is a calcium channel blocker (CCB) frequently used for hypertension [
1]. In the obstetric field, nicardipine is used not only to treat severe hypertension in pregnancy [
2] but also to manage threatened preterm labor [
3]. Spontaneous contraction of the uterus is regulated by intrauterine calcium concentration, and intracellular free Ca
2+ acts as a primary regulator [
4]. Therefore, it is obvious that nicardipine decreases uterine contractility because it acts as an antagonist of calcium influx through the slow channel of the cell membrane [
1]. However, there are few reports about the effective plasma concentration of nicardipine to prevent preterm labor or to treat pregnancy-induced hypertension (PIH), and the relationship between plasma concentration of nicardipine and uterine contractility.
We conducted this study to investigate the relaxant effects of nicardipine on the isolated uterine smooth muscle of the pregnant rat. Furthermore, we aimed to compare the effective concentration for the clinical use of nicardipine in obstetric anesthesia and calculate the effective concentration.
MATERIALS AND METHODS
The current study was conducted after approval of the Animal Care and Use Committee (no. CIACUC2018-S0043) and followed the ARRIVE guidelines [
5]. Female Sprague–Dawley rats (18th day of pregnancy, weighing 200–250 g) were used for the study (n = 10). The rat was killed by inhalation of carbon dioxide, and the abdomen was opened immediately through a midline incision. The pregnant uterus was isolated and dissected in a petri dish filled with Krebs solution; which was composed of 118.3 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl
2, 25 mM NaHCO
3, 1.2 mM KH
2PO
4, 1.2 mM MgCl
2, and 11.1 mM glucose [
6]. After removing connective tissues, uterine smooth muscle tissue was dissected into strips of the myometrium (approximately 5 × 10 mm). The uterine smooth muscle strip was mounted in the tissue baths (25 ml of volume) filled with Krebs solution; maintained at 37°C by circulating the heated water in the space between the double walls, and continuously aerated with a gas mixture of 95% oxygen and 5% carbon dioxide; approximate pH was 7.4. One end of the muscle strip was connected to a hook placed on the base of the bath, and the other end of the muscle strip was connected to a hook which was extended from the lever arm of a forcedisplacement transducer (Isometric transducer, 50–7905, Havard App Ltd., USA).
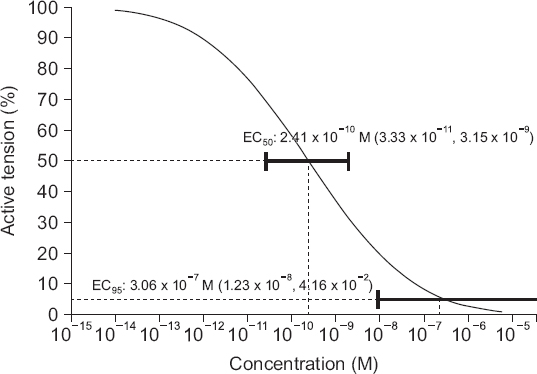
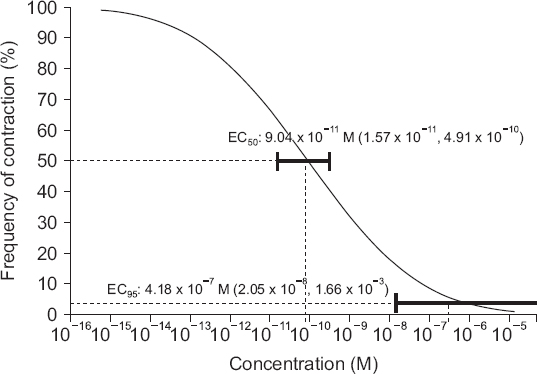
The isometric tension of the myometrial strips was measured using the force displacement transducer and the recorded the traces with an i-WORX 118 system (USA) interfaced via Labscribe software (Windows ver. 2.0, i-Worx Systems Inc.). After applying an initial resting tension of 2.0 g, the uterine smooth muscle strips were equilibrated for 60 min in the bath by flushing fresh solution every 20 min. After the development of rhythmic spontaneous contractions, oxytocin 10 mU/ml was added to the bath and the strips were allowed to equilibrate for a further 60 min. After strong contraction had been accomplished, the parameters of the contraction during the 20 min before application of nicardipine were used as controls. Then, nicardipine (Perdipine, Donga, Korea) in various concentrations (10−12 to 10−8 M) was added to the bath accumulatively every 20 min, and the change of the contraction pattern was observed. Quantitative changes of uterine contractility were expressed by assessing two parameters: active tension (an increase of tension between peak tension and resting tension during muscle contraction), and frequency of contraction (the number of contractions during 20 min for the application of each concentration of nicardipine).
All obtained results are expressed as mean ± standard deviation. The inhibitory effects of nicardipine according to the concentration was assessed by comparing the control and measured data, which were described as a percentage. The statistical significance was analyzed by the Kruskal–Wallis test and Mann–Whitney
U test with Bonferroni’s correction using SPSS (Windows ver. 21.0, SPSS Inc., USA). P values less than 0.05 were considered statistically significant. Effective concentration (EC) of nicardipine needed to inhibit active tension or frequency of contractions of the myometrium was calculated using a probit analysis [
7], and described as it as EC
5 (effective concentration of 5% reduction), EC
25, EC
50, EC
75, and EC
95 (95% confidence interval, CI).
DISCUSSION
Uterine contractility is generated by contractions of myometrial smooth muscle cells [
8]. Contraction of the myometrial smooth muscle is initiated by depolarization of the cell membrane triggered by the entry of Ca
2+ into the cells. An influx of Ca
2+ into the cells contribute to a rise in the intracellular Ca
2+ concentration, and an increase in Ca
2+ concentration leads to a chain reaction of contractions of uterine smooth muscles. Oxytocin stimulates uterine contractions by activating the oxytocin receptor, which triggers elevation of the intracellular Ca
2+ concentration [
9]. Therefore, the concentration of Ca
2+ plays a key role in the contraction of the uterus.
Nicardipine is a CCB that inhibit the influx of Ca
2+ into the cells [
1]. Usually, it is used to treat hypertension, congestive heart failure, and cerebrovascular disease. However, it is also used in the obstetric field for PIH [
2] or preterm labor [
3]. Because nicardipine reduces intracellular Ca
2+, use of nicardipine in pregnancy can decrease uterine contractility. Nicardipine is an effective drug for the treatment of PIH or preeclampsia [
2,
10], and the relaxation effect on the uterus is useful in treating preterm labor [
3]. However, the effect of nicardipine on uterine contractions according to the concentration is still questionable. There are few reports about effective plasma concentration in inhibiting the contraction of the uterus in preterm labor, and besides nicardipine to treat PIH may be associated with uterine atony after delivery by Cesarean section. Thus, we wanted to compare the plasma concentration in the clinical use of nicardipine and estimated plasma concentration by means of an in vitro study.
In human study of nicardipine treatment of PIH, maternal plasma concentration of nicardipine was 54.6 ng/ml after intravenous infusion (2–6 mg/h) [
10], which is 1.14 × 10
−3 M. This plasma concentration is about 2.28 × 10
−5 M in vitro, because 98% of nicardipine given intravenously is bound to plasma protein [
11]. Maternal nicardipine concentrations of the pregnant with severe pre-eclampsia who were treated with intravenous infusions of nicardipine (3–10 mg/h) ranged from 9.8 to 116 ng/ml, which is 2.04 × 10
−4 M to 2.42 × 10
−3 M [
11]. This plasma concentration is about 4.08 × 10
−6 M in vitro. The EC
95 of nicardipine that inhibits the active tension and frequency of contraction of the uterine smooth muscle in the current study was 3.06 × 10
−7 M (95% CI [1.23 × 10
−8 M, 4.16 × 10
−2 M]) and 4.18 × 10
−7 M (95% CI [2.05 × 10
−8 M, 1.66 × 10
−3 M]), respectively. Thus, nicardipine, which is commonly used clinically for the treatment of PIH or pre-eclampsia, would decrease uterine contraction. In fact, the plasma concentration of nicardipine decreases rapidly after an intravenous infusion because of its initial rapid distribution halflife (2.7 min) [
12]. As far as we know, there is still no report of uterine atony associated with nicardipine use for PIH or preeclampsia. The concentration of nicardipine seems to fall fast because of its short half-life, and the use of nicardipine in the pregnant with hypertension would be safe in terms of uterus contraction after delivery. However, it seems be possible to reduce the dosage of nicardipine in the treatment of PIH according to the results of this study.
On the other hand, the use of nicardipine to prevent preterm labor needs a sufficient concentration of plasma nicardipine to relax the uterus. As far as we know, there is still no report about the maternal plasma concentration of nicardipine. However, peak maternal nicardipine concentrations ranged from 175 to 865 ng/ml according to the animal study using rhesus macaques [
13]. This plasma concentration is about 3.65 × 10
−3 M to 1.80 × 10
−2 M, which are 7.30 × 10
−5 M to 3.61 × 10
−4 M in vitro. These concentrations are larger than the EC
95 for relaxing and decreasing the frequency of contraction of the uterine smooth muscle in this study which were 0.6 × 10
−7 M and 4.18 × 10
−7 M, respectively. Another in vitro study about the inhibition of uterine contractility by nicardipine showed EC
50 and EC
75 for the inhibition were 5.8 × 10
−9 M and 2.6 × 10
−8 M, respectively [
14]. EC
50 and EC
75 for the inhibition of uterine contraction of this study were 2.41 × 10
−10 M and 4.51 × 10
−9 M, respectively. The uterine relaxations were achieved at the lower concentrations in our study. It seems that the discrepancies between our results and those of previous animal studies resulted from the differences in methodology (solutions, measurement apparatus, time interval, basal tension, calculating method for estimated EC, etc.). However, the plasma concentrations of nicardipine which are used for preterm labor seem to be sufficient to decrease contractility and the frequency of contraction of the uterine smooth muscle.
According to the results of our study, it seems that it may be possible to decrease the dosage of nicardipine in the treatment of PIH or preterm labor. The concentrations of nicardipine in the PIH treatment of previous clinical studies [
10,
11] or in the treatment of preterm labor in animal studies [
13,
14] are much higher than the calculated concentrations of our study. Nicardipine treatment as a tocolytic has side effects, such as hypotension or maternal pulmonary edema [
3]. Especially, pulmonary edema after use of nicardipine is mainly associated with preterm labor, and it is a severe complication for which the patients needed hospitalization in an intensive care unit [
15]. Thus, limiting the dosage to what is needed to achieve the effective concentration for uterine relaxation and to avoid maternal complications is important. However, validations are needed to extrapolate the results of this study for the clinical use because of possible species differences and in vivo/vitro differences, and this is the limitation of our study. Another well-designed clinical study is needed to find out the minimal effective concentration of nicardipine in the treatment of preterm labor.
In conclusion, nicardipine (from 10−12 to 10−8 M) relaxed and decreased the frequency of contraction of the uterine smooth muscle as a concentration-dependent pattern. The EC95 for decreasing the active tension and the frequency of contraction were 3.06 × 10−7 M and 4.18 × 10−7 M, respectively. It may be possible to adjust the clinical dosage of nicardipine in the obstetric field based on the results of this study. Further clinical studies are needed to confirm our results.