CASE REPORT
The patient was a 43-year-old woman with no relevant medical history, with the exception of glaucoma and migraine. She visited our emergency room due to severe headache at both parietal lobes, which began the evening before the visit. Her headache was caused by sitting, standing, or walking, and was relieved by lying down. She also complained of dizziness; however, no nausea or vomiting was reported. She had a medical history of migraine episodes, which occurred approximately 5–6 times per year. After administration of intravascular tramadol, a non-steroidal antiinflammatory drug, and hydration in the emergency room, she exhibited signs of improvement and, accordingly, was discharged from the hospital and visited the neurology department the following day.
Despite 3 months of prescribed medication and outpatient treatment, her headache progressively worsened. In the neurology department, headache caused by dehydration and intracranial hypotension was suspected, and the pain clinic was consulted for an epidural blood patch. The epidural blood patch was performed by injecting 20.0 ml of autologous blood via an interlaminar approach between C7 and T1, which is the most common leakage site of SIH [
3]. The patient’s symptoms improved from 8/10 to 3/10 on a numerical rating scale (NRS [0 points: no pain, 10 points: the most severe pain imaginable]); however, the symptoms reverted to NRS 8/10 6 days after the intervention. MR myelography revealed multiple CSF leaks from C4 to T3, and on the left T5 to T6 along the nerve root sleeve (
Fig. 1). Repeated epidural blood patch treatments were performed, with 12.0 ml of autologous blood injected in the interlaminar space between C6 and C7, and between T1 and T2. After the procedure, the patient’s orthostatic headache was relieved from 8/10 to 5/10 on the NRS. Two additional epidural blood patch treatments were performed after the procedure: 15.0 ml of autologous blood was injected between C7 and T1, and 10.0 ml of autologous blood was injected between T5 and T6. After 3 blood patch treatments, the patient’s orthostatic headache was relieved, with a change in the NRS score from 8/10 to 3/10, and she was discharged.
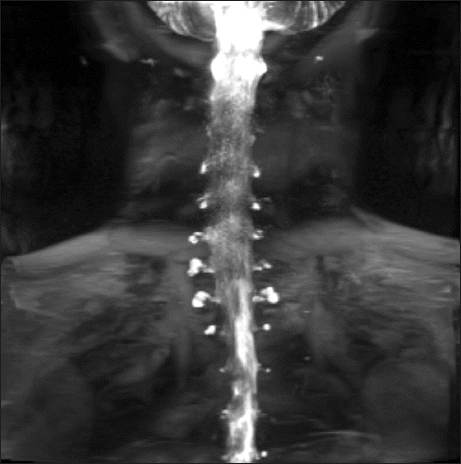 | Fig. 1In the myelography performed after hospitalization, multiple cerebrospinal fluid leaks from C4 to T3 and left T5–6 along the nerve root sleeve were detected. 
|
Twelve days after discharge, she was readmitted as her headache worsened. Brain computed tomography was performed to identify the etiology but did not reveal any additional abnormalities. Epidural blood patch treatments using interlaminar and transforaminal approaches were performed for multiple CSF leaks from C4 to T3 and on the left T5 to T6 along the nerve root sleeve. Using the T2 transforaminal approach, 12.5 ml of autologous blood was bilaterally injected. Two further epidural blood patch treatments were performed during the hospitalization period and, after the procedure, the patient’s pain was controlled (NRS 2–3/10) and she was discharged.
During the subsequent 4 months, the patient underwent 9 additional epidural blood patch treatments with 3 readmissions, and experienced cycles of improvement and recurrence of symptoms. Repeated MR myelography after epidural blood patch treatment resulted in only minimal improvement of multiple CSF leaks (
Fig. 2). C-spine MR imaging to identify the cause did not reveal any additional abnormality, with the exception of mild herniation and protrusion of discs C4 to C6. Blood tests performed repeatedly during hospitalization also revealed no abnormality. Despite 15 epidural blood patch treatments using multiple approaches, including interlaminar, transforaminal, and a combination of both, the beneficial effects did not persist. We searched for reports of similar cases in order to improve the patient’s symptoms, including case reports describing the use of fibrin glue instead of blood in the epidural patch.
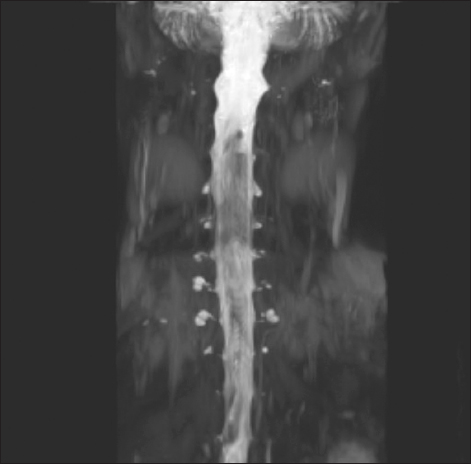 | Fig. 2In the myelography performed prior to the fibrin glue epidural patch treatment, multiple cerebrospinal fluid leaks from C4 to T3 along the nerve root sleeve were detected. 
|
After the associated costs and possible complications were explained to the patient, she opted to undergo fibrin glue patch treatment. Pretreatment with steroids (intravenous dexamethasone 5 mg) was performed 1 day prior to the procedure to prevent the occurrence of allergic complications caused by the use of fibrin glue. After 20 gauge (G) epidural needle insertion via a paramedian interlaminar approach between C7 and T1, contrast medium was injected to ensure the needle was correctly positioned, and then Tisseel 4.0 ml (fibrinogen 184 mg, Baxter, USA) was injected bilaterally via the epidural needle. Then, 23 G spinal needles were inserted via a transforaminal approach at the bilateral T1–2 and T2–3 foraminal space, and Tisseel 2.0 ml was injected via each needle (
Fig. 3). Three days after the procedure, the patient’s orthostatic headache remained below NRS 3/10. After discussion with the patient, for complete improvement, 2 additional epidural fibrin glue patch treatments were performed with the patient’s consent. The patient was discharged after improvement of orthostatic headache after the 3 fibrin glue patch treatments, and she has not reported postural headache for > 1 year.
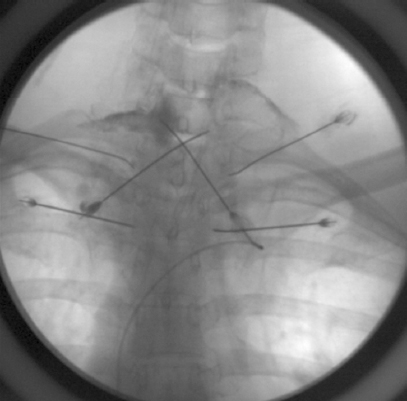 | Fig. 3An interlaminar approach was used between C7 and T1, and a transforaminal approach was used between T1 and T2, and T2 and T3; the location was confirmed by injection of a contrast medium. 
|
Go to :

DISCUSSION
The present case report is the first to describe epidural fibrin glue patch treatment in Korea, and the first report of multiple fibrin glue patch treatments for complete relief.
SIH is a relatively rare condition, with a prevalence of 5 per 100,000 persons. SIH is most common in women in their fifth decade of life who have a history of migraine [
1]. The woman in the present case was 43 years of age with a medical history of migraine. Treatment for SIH includes bed rest, hydration, caffeine intake, steroid use, and use of epidural blood patches [
4]. The patient in the present case experienced no effect while on conservative management in the neurology department and was referred to a pain clinic for epidural blood patch treatment. So et al. [
5] reported that an average of 1.11 ± 0.35 epidural blood patches was needed to resolve iatrogenic post-dural puncture headache, and an average of 1.48 ± 0.64 epidural blood patches was needed to resolve SIH. They stated that the presence of multiple leakage sites explain why a greater number of epidural blood patches were needed to treat SIH than iatrogenic post-dural puncture headache. In our case, 15 high-volume epidural blood patch treatments were performed as part of an overall treatment strategy, which, unfortunately, resulted in repeated deterioration after transient improvement.
The 15 epidural blood patches we attempted were as follows: the 1st epidural blood patch was a C6–7, interlaminar approach, 20.0 ml; 2nd was a C6–7, interlaminar approach, 12.0 ml and T1–2, interlaminar approach, 12.0 ml; 3rd was a C7–T1, interlaminar approach, 15 ml and T5–6, interlaminar approach, 10.0 ml; 4th was a C6–7, interlaminar approach, 15.0 ml and T4–5, interlaminar approach, 10.0 ml; 5th was a bilateral T2 transforaminal approach, 12.5 ml each; 6th was a T5–6, interlaminar approach, 20.0 ml; 7th was a T1–2, interlaminar approach, 20.0 ml; 8th was a C6–7, interlaminar approach, 10.0 ml and T2–3, interlaminar approach, 10.0 ml; 9th was a right T1–2, paramedian approach, 15.0 ml and left T1–2, paramedian approach, 10.0 ml; 10th was a right C7– T1, paramedian approach, 10.0 ml and left T1–2 paramedian approach, 5.0 ml and right T2–3 paramedian approach, 10.0 ml; 11th was a bilateral C7–T1, paramedian approach, 5.0 ml each and right T2–3, paramedian approach, 5.0 ml and left T5–6, transforaminal approach, 5.0 ml; 12th was a right T1–2, paramedian approach, 10.0 ml and left T2–3, paramedian approach, 10.0 ml; 13th was a left T1–2, paramedian approach, 6.0 ml and right T2–3, paramedian approach, 10.0 ml; 14th was a bilateral C7–T1, transforaminal approach, 10.0 ml each; and 15th was a left T1–2, paramedian approach, 15.0 ml and right T1–2, paramedian approach, 7.0 ml.
There have been 6 case reports in which epidural fibrin glue patch treatment has been used to resolve almost intractable and postural headaches due to various causes (
Table 1) [
4,
6–
10]. In 2 cases reported by Mammis et al. [
4], epidural blood patches were performed 1 and 3 times, and did not result in any improvement; however, we were able to completely resolve the postural headache using an epidural fibrin glue patch. In 3 cases reported by Freeman et al. [
6], epidural blood patches were attempted 3, 1, and 0 times; however, the postural headaches were eventually and completely resolved using epidural fibrin glue patches. Kamada et al. [
7] and Gladstone et al. [
8] did not report a significant effect after 2 epidural blood patch treatment attempts in patients with postural headache due to SIH, and eventually resolved the headaches using epidural fibrin glue patches. Schievink et al. [
9] performed at least 3 epidural blood patches per patient, with 1 epidural fibrin glue patch resolving the problem in 2 of 4 patients with postural headache due to SIH. Elwood et al. [
10] performed at least 1 epidural blood patch per patient; however, epidural fibrin glue patches with autologous blood were effective in 4 of 8 patients with postural headache due to SIH. Two patients experienced complete resolution of symptoms, while 2 experienced decreased pain. In all previous reports, the physicians used an interlaminar approach.
Table 1
Previously Reported Cases of Epidural Fibrin Glue Patch Use
|
Source |
Age (yr)/gender |
Indication |
Level |
Dose |
Result |
|
Mammis et al. [4] |
35/Female |
Postural headache after IDDS |
L2–3 |
5.0 ml |
Resolved |
|
49/Female |
Postural headache after SCS |
L2–3 |
10.0 ml |
Resolved, after 2 weeks |
|
Freeman et al. [6] |
43/Female |
Postural headache after IDDS |
L2–3 |
3.0 ml |
Resolved |
|
64/Female |
Postural headache after IDDS |
L2–3 |
10.0 ml + 2.0 ml autologous blood |
Resolved |
|
38/Female |
Postural headache after IDDS |
L1–2 |
7.0 ml |
Resolved, temporary pressure paresthesia |
|
Kamada et al. [7] |
58/Female |
Postural headache due to SIH |
C2 |
2.8 ml |
Resolved, after 36 hours |
|
Gladstone et al. [8] |
44/Female |
Postural headache due to SIH |
T2–3 |
7.5 ml |
Resolved, after 24 hours |
|
Schievink et al. [9] |
43/Male |
Postural headache due to SIH |
T12–L1 |
4.0 ml |
Resolved |
|
39/Female |
Postural headache due to SIH |
C5–6–7 |
16.0 ml |
Poor effect |
|
26/Male |
Postural headache due to SIH |
C4–5–6–7 |
20.0 ml |
Resolved |
|
43/Male |
|
C6–7 |
10.0 ml |
Poor effect |
|
Elwood et al. [10] |
Female |
Orthostatic, exertional, and Valsalva headache |
L1–2 |
|
No effect |
|
Female |
Postural headache due to SIH |
L1–2 |
10.0 ml autologous blood + up to 10.0 ml fibrin glue |
No effect |
|
Female |
Postural headache due to SIH |
L1–2 |
|
Partially resolved |
|
Male |
Postural headache due to SIH |
L1–2 |
|
No effect |
|
Male |
Postural headache due to SIH |
L1–2 |
|
Partially resolved |
|
Female |
Postural headache due to SIH |
L1–2 |
|
Partially resolved |
|
Male |
Postural headache due to SIH |
L1–2 |
|
Partially resolved |
|
Female |
Postural headache due to SIH |
L1–2 |
|
Resolved |

In previous reports describing the use of fibrin glue patches, all cases required only a single treatment. However, our case was atypical and unique, in that the patient experienced headache relief after 3 epidural fibrin glue patch treatments, despite the ineffectiveness of 15 high-volume epidural blood patches. In the cases described by Schievink et al. [
9] and Elwood et al. [
10], some patients exhibited partial or no effects from the epidural fibrin glue patch or deteriorated after improvement. In such cases, we can expect that multiple fibrin glue patch treatments, similar to those in our case, may resolve–or at least improve–patient symptoms completely. We believe that some patients with intractable SIH may require multiple fibrin glue patch treatments for complete relief.
In our case, 3 epidural fibrin glue patch treatments using interlaminar and transforaminal approaches were used to resolve the postural headache. These 3 treatments were as follows: the 1st epidural fibrin glue patch was a bilateral T1–2, paramedian approach, 4.0 ml each and bilateral T2–3 and right T4, transforaminal approach, 2.0–4.0 ml each; 2nd was a bilateral T1–2, paramedian approach, 4 ml each and bilateral T3–4, paramedian approach, 4.0 ml each; and 3rd was a bilateral T1–3, transforaminal approach, 2.0 ml each and bilateral C7–T1, paramedian approach, 4.0 ml each.
In SIH, many CSF leakage sites involve multiple root sleeves. Therefore, we believe that the transforaminal approach may be more effective than the interlaminar approach for spreading to root sleeves. Further studies comparing interlaminar and transforaminal epidural patches in SIH are warranted.
Fibrin glue is generated from plasma. Fibrinogen, factor XIII, fibronectin, aprotinin, and plasminogen constitute 1 solution, and thrombin and calcium constitute the other. When fibrin glue is injected, the 2 solutions are mixed. The mixed solution stimulates the final step of the coagulation cascade, thereby converting fibrinogen to fibrin and factor XIII crosslinking fibronectin. As a result, a fibrin clot is formed, which binds to collagen and attaches to the leak where it acts as a powerful adhesive [
6].
There are several advantages to the epidural fibrin glue patch. It is harder than coagulated autologous blood and has a relatively low capacity for injection; therefore, coagulation occurs rapidly. It is less complicated to perform than the epidural blood patch, and it decomposes if unintentionally injected into the spinal cord. The epidural fibrin glue patch can be performed in patients with poor-quality blood vessels and in whom blood collection is difficult [
11].
There are also disadvantages to the epidural fibrin glue patch. Because fibrin glue is made from plasma, it is likely to cause infection. There is also the possibility of hypersensitivity reactions, such as anaphylaxis; however, the incidence of hypersensitivity to aprotinin, which can induce anaphylaxis, is relatively low (5/100,000 persons) [
12]. There is also the possibility of embolization after intravascular administration of fibrin glue. However, the epidural fibrin glue patch can reduce the risk of intravenous administration by confirmation with a contrast agent in advance. Another disadvantage is that the high cost can place an economic burden on the patient [
11]. According to our experience, narrow-diameter needles (i.e., 23 G) can easily be clogged by fibrin glue. If fibrin glue is not injected rapidly, 23 G needles can become clogged before injection of 2.0 ml of fibrin glue. Therefore, for injection the epidural fibrin glue patch, it is necessary to perform the injection rapidly, before the drug hardens. A narrow needle may require greater attention because the drug can harden easier and faster.
An indication of the epidural fibrin glue patch is that the epidural blood patch was performed in a patient with SIH, but the symptoms recurred or did not improve. However, a contraindication of the epidural fibrin glue patch is the occurrence of infection of the injection site, septic condition, coagulopathy, and patient rejection.
There are limitations associated with the use of epidural fibrin glue patches in Korea. The indications for epidural fibrin glue patches include only local hemostasis, closing, and tissue adhesions. The epidural fibrin glue patch is not yet indicated for standard use in Korea.
Fibrin glue is currently widely used in orthopedic surgery and neurosurgery to facilitate wound sealing and hemostasis in the operating room [
13]. The epidural fibrin glue patch was first described by Gerritse et al. [
14] in 1997, but is not yet adequate to be widely used as a general treatment, and further studies and case reports are needed.
Treating postural headache associated with SIH using an epidural fibrin glue patch appears to be associated with more benefits than risks. In addition, this case and others have demonstrated its efficacy for intractable orthostatic headache. We believe that the epidural fibrin glue patch may be beneficial for the treatment of refractory postural headaches caused by SIH.
Go to :





 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download