INTRODUCTION
The Vigileo-FloTrac® system (Edwards Lifesciences, USA), founded on the analysis of arterial pressure wave, is used for automatic and continuous monitoring of cardiac output and stroke volume variation (SVV). The reliability of this system to measure cardiac output has been established in numerous settings with various results [1–7]. In addition, SVV measured by this device has been found to be capable of predicting fluid responsiveness in patients with mechanical ventilation [8–12].
In thoracic surgery, lung resection and/or one lung ventilation (OLV) may cause postoperative acute lung injury [13–15]. In such cases, fluid management is important. Intraoperative fluid overloading is a significant predictor of postoperative lung injury [16,17]. Fluid restriction is usually recommended for lung surgery [17–19]. Thoracotomy (open thorax) and OLV can lead to changes in airway and intrathoracic pressure. Because SVV is based on cyclic changes of intrathoracic pressure during the respiratory cycle, the usefulness of SVV to predict fluid responsiveness is conflicting under the open chest conditions [20–24]. However, previous studies have reported that SVV can predict fluid responsiveness in patients during OLV under thoracoscopy [25,26].
Most studies for open chest and/or OLV have focused on the usefulness of SVV for predicting fluid responsiveness. However, no study has reported whether thoracotomy or OLV affects changes of SVV when the fluid volume is sufficient. Thus, the aim of this study was to determine whether thoracotomy and converting from two lung ventilation (TLV) to OLV might affect values of SVV and the hemodynamic change using Vigileo-FloTrac® system.
MATERIALS AND METHODS
This study was approved by the institutional review board of Chonbuk National University Hospital (no. 2011-01-007). All patients provided written informed consent before surgery. Thirty patients aged 20 to 65 years who were scheduled for pulmonary lobectomy or pneumonectomy requiring OLV were enrolled in this study. Exclusion criteria were: American Society of Anesthesiologists physical status ≥ III, cardiac arrhythmia, or severe obesity with a body mass index ≥ 35.
Electrocardiography, pulse oximetry, and noninvasive blood pressure monitoring were applied upon arrival in the operating room. Induction and maintenance of anesthesia were performed with propofol (target concentration of 3–5 μg/ml) and remifentanil (3–5 ng/ml) via total intravenous anesthesia, and the depth of anesthesia was maintained at bispectral index (BIS) of 40–55 using a BIS monitor (BIS VISTA™, Aspect, USA). Endotracheal intubation with leftsided double-lumen tube (Mallinckrodt™, Covidien, USA) was facilitated by rocuronium (1.0 mg/kg), and the location of double-lumen tube was confirmed using a fiberoptic bronchoscope. An arterial catheter was inserted in the left or right radial artery. Cardiac output and SVV were measured using a Vigileo-FloTrac® system (Vigileo™, Edwards Lifesciences).
Patients’ lungs were mechanically ventilated with a tidal volume of 8 ml/kg (ideal body weight) during both TLV and OLV. The inspired oxygen fraction was 0.5 during TLV and 1.0 during OLV. The inspiratory to expiratory time ratio was 1 : 2. The inspiratory pause time was 20% while the positive endexpiratory pressure (PEEP) was 3 cmH2O (intrinsic PEEP of circuit). The respiratory rate was adjusted to maintain endtidal carbon dioxide tension at 30 to 35 during TLV and 35 to 40 mmHg during OLV.
After induction of anesthesia, patient’s position was changed to lateral decubitus. To minimize the effect of anesthesia induction on SVV, all patients were administered 3–5 ml/kg of crystalloid fluid intravenously after the induction of anesthesia, and kept 2 ml/kg/h until skin incision. Additional fluid was administered when bleeding was observed or when it was deemed necessary by attending anesthesiologists. This was proceeded only when a patient met the following criteria: cardiac index (CI) ≥ 2.5 L/min/m2 and SVV ≤ 10%. Hemodynamic variables including heart rate (HR), mean arterial pressure (MAP), CI, and SVV were measured at intervals of 1 min for 10 min after thoracotomy and OLV, respectively. For all subjects, thoracotomy and OLV were carried out. In order to collect hemodynamic variables of thoracotomy itself, surgical procedure was held when the muscle of chest wall was dissected with surgeon’s cooperation. Baseline data were collected after waiting for stabilized vital sign just before thoracotomy. Also, post-thoracotomy data were collected for 10 min after the thoracotomy with stabilized vital sign in the absence of surgical stimuli.
Anesthetic depth was adjusted for stabilizing the vital sign within 20% of preoperative vital signs. When the vital sign was stable for more than 5 min, baseline values were collected and hemodynamic parameters were then recorded for 10 min after thoracotomy without surgical stimulus. Hemodynamic variables from TLV to OLV were also collected in the absence of surgical manipulation. Tidal volume during OLV was kept at 8 ml/kg (based on ideal body weight) as TLV, and plateau airway pressure was collected before and after thoracotomy and OLV.
All statistical analyses were performed using SigmaPlot 12.0 (Systat Software Inc., USA). All data are expressed as mean ± SD and median (1Q, 3Q) except number of patients. Normality test was performed for all variables with the Shapiro–Wilk test. Some hemodynamic variables violated the normality. These variables were evaluated with repeated measures analysis of variance (ANOVA) on ranks and Dunnett’s method was applied as post-hoc test. Data of airway pressure were analyzed with a paired t-test. Statistical significance was considered at P < 0.05.
RESULTS
Seventeen male and 13 female patients were enrolled in this study. Their mean age, height, and weight were 56.3 ± 13.3 years, 160.7 ± 10.5 cm, and 59.5 ± 10.9 kg, respectively. Since the baseline value of SVV was more than 10% in 5 patients for thoracotomy and 6 patients for OLV, hemodynamic data were analyzed for 25 patients after thoracotomy and 24 patients after OLV.
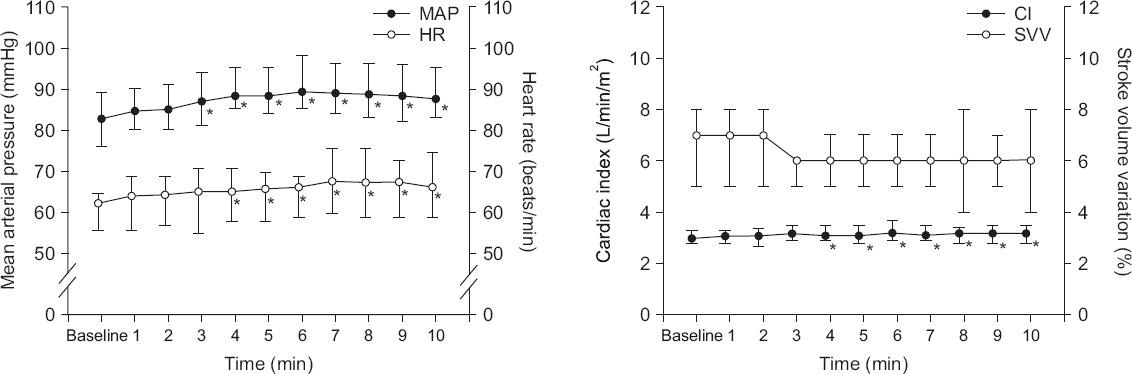
After thoracotomy, MAP and HR increased at 3–10 and 4–10 min, respectively (P < 0.001). CI increased at 4–10 min (P < 0.001). SVV was not changed for 10 min after thoracotomy (P = 0.084, Fig. 1). Plateau airway pressure decreased from 14.5 ± 2.2 to 10.1 ± 3.5 cmH2O after thoracotomy (P < 0.001).
Fig. 1
Change of hemodynamics after thoracotomy. Mean arterial pressure (MAP) and heart rate (HR) increased at 3–10 and 4–10 min, respectively (P < 0.001). Cardiac index (CI) increased at 4–10 min (P < 0.001). Stroke volume variation (SVV) was not changed for 10 min (P = 0.084). Values are presented as medians (1Q, 3Q). *P < 0.05 vs. baseline value.
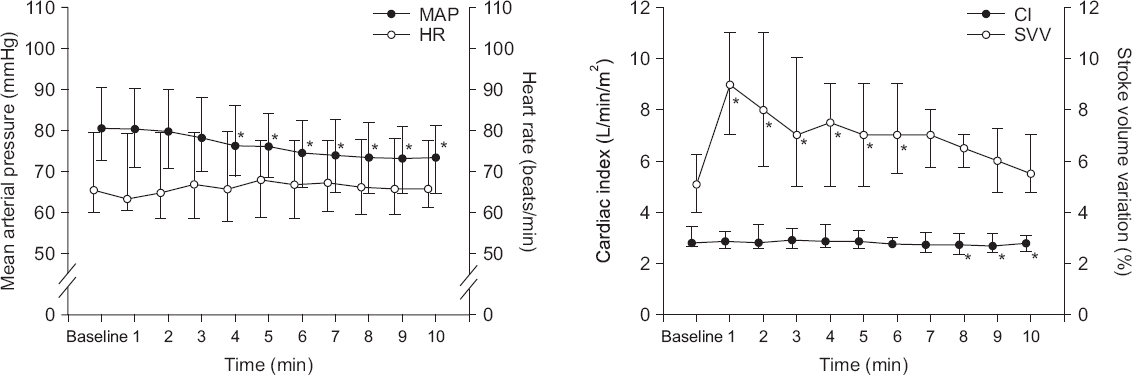
After starting OLV, MAP decreased at 4–10 min (P < 0.001) while HR did not change for 10 min. SVV increased to the highest at 1 min after OLV and returned to the baseline value at 7 min (P = 0.112). SVV temporarily increased above 15% for 4 patients. Cardiac index decreased at 8–10 min (P < 0.001, Fig. 2). Plateau airway pressure increased from 11.4 ± 2.7 to 17.9 ± 4.4 cmH2O after OLV (P < 0.001).
Fig. 2
Change of hemodynamics after one lung ventilation. Mean arterial pressure (MAP) decreased at 4–10 min (P < 0.001) while heart rate (HR) did not changed (P = 0.112). Stroke volume variation (SVV) increased to the highest at 1 min and returned to the baseline value at 7 min. Cardiac index (CI) decreased at 8–10 min (P < 0.001). Values are medians (1Q, 3Q). *P < 0.05 vs. baseline value.
DISCUSSION
The main finding of this study was that cardiac output was mildly increased after thoracotomy whereas it was gradually decreased during OLV. SVV obtained from a Vigileo-FloTrac® system temporarily increased after OLV and not changed in thoracotomy. This temporary increase of SVV after change from TLV to OLV should be considered when fluid responsiveness is predicted by SVV using a Vigileo-FloTrac® system during the early period after starting OLV.
SVV is based on the interaction between heart and lung by the cyclic changes of intrathoracic pressure. At the inspiratory phase, increased alveolar pressure leads to purge of pulmonary venous blood into the left heart that can increase left ventricular stroke volume [25]. However, increased intrathoracic pressure can decrease venous return to the right heart and increase afterload of the right ventricle. Approximately three heartbeats later, this decrease in stoke volume of the right ventricle can result in a decrease in the left ventricular stroke volume at the expiratory phase [8,26]. Cannesson et al. [8] have reported that a threshold SVV value of 10% allows discrimination of responders to volume loading with a sensitivity of 82% and a specificity of 88%.
In this study, before thoracotomy and OLV, fluid was administered sufficiently for volume deficiency such as vasodilatation, bleeding, and third space loss. The protocol proceeded only in patient who had over five minutes of hemodynamic stability, cardiac index ≥ 2.5 L/min/m2, and SVV ≤ 10% under no surgical manipulation. To avoid hypovolemia as much as possible, the criterion of SVV was set at 10%. After thoracotomy, MAP and CI increased while SVV did not change in our results. Theoretically, a relatively small change in intrathoracic pressure by open thorax during inspiration does not interrupt systemic venous return (preload) to right heart while the left ventricular stroke volume during expiration is preserved. Therefore, an increase in minimum stroke volume can result in a decrease in SVV value. However, SVV was not changed in our study because the baseline value might be less than 10%. According to De Blasi et al. [27], pulse pressure variation (PPV) was decreased after sternotomy for patients whose PPV was more than 8% while PPV was unchanged for patients whose PPV was less than 8%. Decreases in intrathoracic pressure can increase preload of the right heart and decrease afterload, thus increasing MAP and CI.
After starting OLV under thoracotomy, the value of SVV measured with a Vigileo-FloTrac® system was temporarily increased. It then returned to the baseline value. During OLV, ventilated and non-ventilated lungs might have changed differently. When tidal volume was kept and OLV was started, increased transpulmonary pressure purges pulmonary venous blood into the left heart in ventilated lung. On the other hand, such effect of the purge of pulmonary venous blood does not exist in non-ventilated lung. As a blood flow toward the dependent lung in lateral position is increased by gravity, the effect of dependent lung might be greater than the other, thus increasing the maximum SV. When OLV was started, MAP and CI decreased as time passed during the operation. According to Kozian et al. [15], cardiac output decreases in pig under thoracotomy and OLV due to non-ventilated lung collapse, hypoxic pulmonary vasoconstriction, and increased afterload of the right ventricle by increased airway pressure in ventilated lung. It is challenging to explain the cause of decrease of SVV to the baseline.
In most recent cases, tidal volume is set at 6 ml/kg with 5 cm H2O of PEEP during OLV. However, tidal volume was set at 8 ml/kg in this study. Suehiro and Okuni [28] have proposed that SVV could predict fluid responsiveness in OLV only when tidal volume is at least 8 ml/kg. Therefore, we did not apply PEEP to remove the hemodynamic influence of PEEP.
The use of SVV or dynamic variables is increasing for fluid management of perioperative or intensive care unit patients with ventilation. A Vigileo-FloTrac® system via arterial pressure wave have advantages because cardiac output can be monitored automatically and continuously with Vigileo-FloTrac®, but not with echocardiogram or aortic Doppler. In addition, it is less invasive than pulmonary catheterization. With these advantages, this device may be utilized as a bedside point-of-care device. However, SVV is affected by tidal volume, respiratory rate, and intrathoracic pressure [29,30] and the absolute value of cardiac output is less accurate when systemic vascular resistance (SVR) is decreased [11] or under hyperdynamic condition [2]. Although SVR was not measured in this study, changes in SVR due to anesthetic depth and effects of anesthetics may alter the effective circulating volume. Thus, clinicians should be cautious when interpreting cardiac output and SVV obtained from the arterial pulse wave. In the present study, although there was no decrease in actual intravascular volume, there was a temporary increase in SVV during OLV.
The present study has some limitations. First, we did not measure the cardiac output from a pulmonary artery catheter or echocardiogram. However, a Vigileo-FloTrac® system has been a clinically acceptable device to measure cardiac output [1–5]. In addition, cardiac index and SVV were within normal ranges during the study. Second, because the present study was conducted during surgery, changes of hemodynamic variables might not be due to thoracotomy and OLV absolutely. Although we have tried to exclude surgical stimuli during this study, it may be possible to make hemodynamic depression by anesthesia. However, this is a similar problem in most clinical studies have. Third, because similar previous studies were unavailable, the sample size was randomly calculated to be 30 patients. Therefore, the results of this study may be characteristics of a preliminary study. Further study with a strict sample size calculation is needed. Fourth, hypoxic pulmonary vasoconstriction (HPV) in OLV is an important mechanism that controls the pulmonary shunt. Parameters that might affect HPV were not included in this study. However, desaturation under 96% of pulse oximetry did not occur in this study. Lastly, we used less conservative post hoc test for statistical analysis because this was the first study to figure out the effect of thoracotomy and OLV on SVV changes in patients with adequate fluid volume based on SVV values monitored with Vigileo-FloTrac®. Since our statistical analysis cannot control for the occurrence of type I errors clearly, more conservative comparative methods are needed for subsequent studies.
Most studies for open chest and/or OLV have focused on the usefulness of SVV for predicting fluid responsiveness. Unlike previous studies proving that SVV can predict fluid responsiveness, the present study is focused on the effect of thoracotomy or OLV itself on changes of SVV when fluid volume is sufficient.
In conclusion, SVV measured by a Vigileo-FloTrac® system is increased after OLV temporarily. Such a transient increase of SVV may be considered when fluid responsiveness is predicted by SVV during early period after OLV. Therefore, fluid status with only SVV should not be predicted.
ORCID
Hyungsun Lim: https://orcid.org/0000-0002-6379-9302
Dong-Chan Kim: https://orcid.org/0000-0002-6881-126X
Myung-Jong Kim: https://orcid.org/0000-0002-0031-2253
Seonwoo Yoo: https://orcid.org/0000-0002-1742-7487
Min-Jong Ki: https://orcid.org/0000-0001-9959-7908
Sehrin Kang: https://orcid.org/0000-0001-8211-3831




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download