INTRODUCTION
Spinal anesthesia is the standard anesthetic technique for performing Cesarean sections (C-section) to prevent complications related to airway management during general anesthesia, such as intubation failure or respiratory aspiration [1]. However, spinal anesthesia lowers peripheral vascular resistance by blocking sympathetic nerves, which can cause maternal hypotension [2,3].
Maternal hypotension induces unpleasant symptoms, such as dyspnea, nausea, and vomiting caused by reduced blood flow to the brain. When severe hypotension persists, it may lead to serious complications, such as loss of consciousness, cardiovascular collapse, and ischemia in organs. Furthermore, prolonged maternal hypotension may cause reduced uterine placental blood flow and fetal distress, which may result in bradycardia, hypoxia, and acidosis in the fetus [4,5]. Therefore, it is important to predict the onset of and prevent maternal hypotension.
Several studies have reported that heart rate variability [6,7], the pleth variability index [8], baseline heart rate (HR) [9,10], cerebral oxygen saturation [11], and pulse transit time [12] can be used to predict the onset of maternal hypotension during C-sections under spinal anesthesia. However, it is difficult to measure these values in the recovery or operating rooms immediately before surgery because they require the use of specific programs and instruments that are often unavailable in these locations. Furthermore, studies have shown that the autonomic function test is more sensitive and specific than vasomotor tests, such as measuring total pulse amplitude, when diagnosing diabetic autonomic dysfunction [13]. It was hypothesized that the incidence of maternal hypotension during C-sections under spinal anesthesia was associated with autonomic nervous system function, so this study investigated the correlation between autonomic dysfunction and incidence of maternal hypotension using an autonomic function test which can be performed by simply monitoring maternal blood pressure and HR.
MATERIALS AND METHODS
This study was approved by the institutional review board and informed consent was obtained from all participants (no. DFH17MRIO355).
The subjects in this study were patients undergoing non- urgent C-sections under spinal anesthesia who were American Society of Anesthesiology physical status class I or II and who had gestational ages of 36–41 weeks. Patients undergoing emergency surgery, with twins, with pre-eclampsia, or who took steroids, diuretics, caffeine, alcohol, anticholinergic agents, cholinergic agents, antidepressants, antihistamines, or sympathomimetic drugs were excluded from the study. The participants fasted for eight hours prior to the autonomic function test and urinated naturally.
Of the 35 subjects examined in this study, 3 were with- drawn as they were converted to general anesthesia after spinal anesthesia had failed before C-section was resumed.
First, patients rested in a supine position for 30 min in a quiet room with a neutral temperature and humidity level. Then their systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure were measured noninvasively from the right upper arm and HR was measured using an electrocardiograph. These values were used as controls for the measurements taken during the autonomic function test.
Sympathetic function test
Sympathetic functions were evaluated by observing DBP changes during a hand grip test and SBP changes after standing up. Tests were performed in order to observe HR changes during deep breathing, DBP changes during a handgrip test, and HR and blood pressure changes after standing up [14,15]. Patients rested for 10 min in a supine position between tests.
Hand grip test
During the hand grip test, the difference between peak DBP and DBP immediately before gripping was evaluated. Subjects were instructed to breath at a normal rhythm and depth while gripping with their hands to avoid Valsalva maneuvers. Subjects who had a difference of 16 mmHg or less in blood pressure levels were determined to have tested positive for sympathetic abnormality.
Parasympathetic function test
Parasympathetic functions were evaluated by testing HR changes while taking a deep breath and after standing up. The Valsalva maneuver was not performed to avoid fetal hypoxia [14,15]. Patients rested for 10 min in a supine position between tests.
Deep breathing test
For the deep breathing test, patients were instructed to breathe six times per minute. Each breath was to be a slow inhalation for five seconds and a slow exhalation for five seconds. They were asked not to stop, cough, laugh, talk, or move while breathing and to breathe strongly to reach maximum tidal volume. Patients who had heart rate variability between inhalation and exhalation, which was the difference between maximum and minimum HR, of 15 or less were considered to have tested positive for parasympathetic abnormality.
Supine-to-standing-position test
Patients were instructed to stand up without assistance within four seconds. HRs before and after standing were compared using the 30/15 ratio in which the longest R-R interval was at beat 30 and the shortest R-R interval was at beat 15. Patients with ratios of 1.04 or less were determined to have tested positive for parasympathetic abnormality. R-R intervals were measured to within 0.1 mm by using an electrocardiogram patient monitoring device (Anesthesia V24C, Agilent, Germany).
Autonomic tests were performed in the recovery room immediately before surgery. Patients who were determined to have tested positive for sympathetic abnormality in both tests were defined as having sympathetic dysfunction and those who had positive results for both parasympathetic function tests were defined as having parasympathetic dysfunction. After the tests, patients were moved to the operating room and spinal anesthesia was induced by inserting a 25-gauage pencan needle into the intrathecal space in the left lateral position through the L3–4 vertebra to inject a mixture of 9 mg of heavy Marcaine (bupivacaine) and 20 μg of fentanyl after which oxygen was supplied at 5 L/min through an oxygen mask with the patient in the supine position. A crystalloid solution was injected at 10 mg/kg/h from the induction of anesthesia until delivery. The dose was reduced to a maintenance dose after delivery.
Until delivery of the neonate or until 20 min after induction of spinal anesthesia, patient HR and blood pressure were measured in one-minute intervals. The interval was increased to five minutes once blood pressure stabilized after delivery. After delivery, the neonate’s five-minute Apgar score was recorded.
A total of 100 μg of phenylephrine [16] was intravenously administered when hypotension occurred, which was defined as SBP decreasing 20% or more below initial SBP measured prior to spinal anesthesia [17]. When bradycardia, defined as a HR of 50 beats/min or less, occurred, 0.5 mg of atropine was administered intravenously. The presence of symptoms such as nausea, vomiting, dizziness, and headache were recorded.
The sensory block level was checked five minutes after inducing anesthesia using an alcohol rub. Length of surgery, duration of anesthesia, and volume of intraoperative blood loss were recorded.
Statistical analysis
Normally distributed data were analyzed with Student’s t-test and non-normal data were analyzed with a Mann–Whitney test. All statistical analyses were performed using IBM SPSS version 21.0 (IBM Co., USA). All data were expressed as mean ± standard deviation or as median (interquartile range).
The area under the receiver operating characteristic curve (AUC), cut-off values, sensitivities, and specificities of each parasympathetic and sympathetic test were analyzed.
The correlations between parasympathetic dysfunction, sympathetic dysfunction, and hypotension and the correlation between the incidence of side effects and use of vasopressor agents were analyzed using cross-correlation analysis at a significance level of P < 0.05.
RESULTS
Nine subjects were determined to have tested positive for sympathetic dysfunction while 23 did not and 12 were determined to have tested positive for parasympathetic dysfunction while 20 did not. A total of 22 subjects (68.7%) developed hypotension.
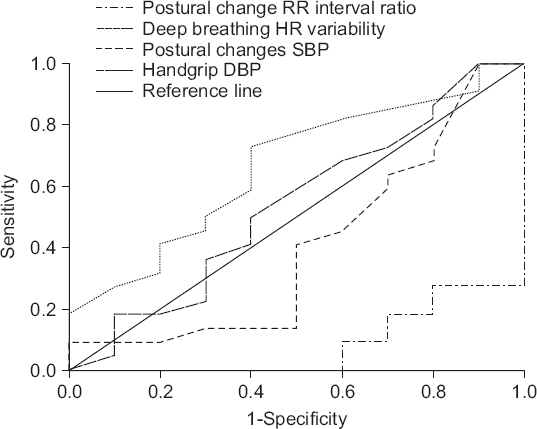
The AUC of the RR interval ratio during the postural change test as a predictor of hypotension and the AUC of systolic blood pressure change during the postural change test as a predictor of hypotension were both below 0.5.
The AUC of the deep breathing test as a predictor of hypotension was 0.664 with a cut-off value of 10.50, a sensitivity of 59%, and a specificity of 60%. The AUC of the hand grip test as a predictor of hypotension was 0.539 with a cut-off value of 7.5, a sensitivity of 45.5%, and a specificity of 40% (Fig. 1).
Fig. 1
Receiver operating characteristic (ROC) curve for each autonomic function tests. Value for area under the ROC curve; RR interval ratio in postural change: 0.082 (95% confidence interval [95% CI], 0–0.176), Heart rate (HR) variability when deep breathing: 0.664 (95% CI, 0.462–0.865), Systolic blood pressure (SBP) in postural changes: 0.405 (95% CI, 0.173–0.636), Diastolic blood pressure (DBP) in handgrip test: 0.539 (95% CI, 0.312–0.765).
There were no significant differences in the demographic data between the patients that were determined to have had sympathetic dysfunction and those that did not (Table 1). The incidences of hypotension in these two groups were 55.6% (5 out of 9 subjects) and 73.9% (17 out of 23 subjects), respectively, indicating that the incidence of hypotension was not significantly correlated with the incidence of sympathetic dysfunction (Table 2).
Table 1
Patient Characteristics (Sympathetic Dysfunction Test Positive Group vs. Negative Group)
Table 2
Hemodynamic Data (Sympathetic Dysfunction Test Positive Group vs. Negative Group)
There were no significant differences in the demographic data between the patients that were determined to have had parasympathetic dysfunction and those that did not (Table 3). However, the incidences of hypotension in these groups were 100% (12 out of 12 subjects) and 50% (10 out of 20 subjects), respectively, indicating that the incidences of hypotension and parasympathetic dysfunction were significantly positively correlated. The frequency of phenylephrine use significantly differed but had no effect on neonates’ five-minute Apgar scores (Table 4).
Table 3
Patient Characteristics (Parasympathetic Dysfunction Test Positive Group vs. Negative Group)
Table 4
Hemodynamic Data (Parasympathetic Dysfunction Test Positive Group vs. Negative Group)
DISCUSSION
Pregnancy induces hemodynamic changes in parturients. During pregnancy, hormonal effects reduce systemic vascular tone and increase vascular volume, which in turn reduce cardiac afterload and increase cardiac preload, thereby increasing cardiac output. Women experiencing normal pregnancy have higher levels of sympathetic activity than those who are not pregnant. This activity is greater in women with gestational hypertension. Unlike sympathetic activity, women experiencing normal pregnancy experience lower levels of peripheral vascular resistance than those who are not pregnant. In late pregnancy, the baroreflex function decreases as a result of increased hormone levels and blood volume and is likely one of the factors that increases sympathetic activity [18].
The AUCs for all of the autonomic function tests were below 0.7, which suggests that they were not adequate predictors of hypotension after spinal anesthesia in pregnant women.
The incidence of hypotension was not significantly correlated with sympathetic dysfunction. Of the 32 subjects, 11 (34.4%) were determined to have had sympathetic dysfunction as a result of the supine-to-standing test and 17 (53.1%) actually showed an increase, rather than an expected decrease, in SBP levels upon standing from a supine position. This result may have been the result of the fact that pregnant women have higher levels of sympathetic activity and vascular capacitance than women who are not pregnant [10] and so experience smaller-than-expected reductions of cardiac output upon standing [19]. Furthermore, sympathetic activity may have been more elevated due to tension and anxiety as the tests were performed immediately before the C-section.
In the hand grip test, DBP was elevated as a result of the baroreflex control and sympathetic activity [20]. An increase in DBP of less than the expected amount of 16 mmHg or more during the hand grip test indicated sympathetic abnormality [21]. In the present study, 29 of the 32 subjects (90.6%) showed a DBP increase of less than 16 mmHg. This result was inconsistent with the fact that pregnant women typically exhibit elevated levels of sympathetic activity, which may have been the result of the fact that pregnant women have lower levels of sympathetic regulation of vascular resistance than women who are not pregnant due to the effects of estrogen [18]. It was hypothesized that most of the participants would be determined to have sympathetic abnormality as a result of the hand grip test because the sympathetic regulation of vascular resistance is already lower in pregnant women without sympathetic activity problems, which would hinder an appropriate rise in DBP after conducting the hand grip test. Another explanation for these results is that pregnant women have reduced baroreflex sensitivity [22].
Unlike the incidence of sympathetic dysfunction, the incidence of hypotension was positively correlated with the incidence of parasympathetic dysfunction. The incidence of hypotension was higher in the parasympathetic dysfunction group than in the non-parasympathetic dysfunction group.
Sun and Huang [8] reported that hypotension after spinal anesthesia is affected by preoperative sympathetic activity and effective circulating blood volume. They explained that hypotension develops as a result of reduced systemic vascular resistance and arterial and venous blood pooling.
In a study analyzing heart rate variability to determine the correlation between hypotension and autonomic dysfunction during C-sections under spinal anesthesia, Hanss et al. [7] reported that the subjects who developed moderate-to-severe hypotension exhibited significantly higher sympathetic and lower parasympathetic drives than those who only had mild hypotension. This result reflects the fact that sympathetic and parasympathetic activity critically affects hypotension during C-section under spinal anesthesia.
In the present study, subjects’ sympathetic drives could not be accurately calculated using autonomic function tests as the hand grip test was believed to be inadequate at demonstrating actual patient sympathetic activity. However, the incidence of hypotension was substantially higher among mothers with parasympathetic dysfunction than those without it. The frequency and average dose of phenylephrine were 2.5 and 266.67 ± 143.55 μg and 0.5 times and 105.0 ± 123.44 μg, respectively, for the same groups. Two subjects who were determined to have parasympathetic dysfunction were even administered phenylephrine five times due to severe hypotension. This result can be explained by the correlation between parasympathetic and sympathetic nervous functions. Women experiencing normal pregnancies have elevated levels of sympathetic activity, so the incidence of parasympathetic dysfunction in hemodynamic subjects can cause a relatively large increase in sympathetic activity. This phenomenon may explain the markedly frequent incidence of hypotension caused by sympathetic nervous block after spinal anesthesia.
Olang et al. [23] and Corke et al. [24] reported that maternal hypotension that persisted for less than two minutes did not have a significant impact on neonates’ five-minute Apgar scores or the incidence of neonatal acidemia. These findings highlight the importance of being able to predict and discover maternal hypotension.
Autonomic tests were conducted immediately before entering the operating room, so the subjects may have had more autonomic activity than pregnant women at rest due to anxiety about the impending C-section. Further, this was a pilot test with an insufficient number of cases to achieve normal distribution, so subsequent studies must be conducted on greater sample sizes. Finally, a significant association between parasympathetic activity and incidence of hypotension during C-section under spinal anesthesia was confirmed based on autonomic function tests, but it was difficult to accurately determine the degree to which each patient’s sympathetic function had decreased.
In conclusion, maternal hypotension after spinal anesthesia during C-section may induce maternal complications and fetal distress syndrome, so it is critical to be able to predict and quickly correct the condition. This study’s findings suggest that parasympathetic function tests can be used as predictors of maternal hypotension.
ORCID
Hyun Kim: https://orcid.org/0000-0003-4994-1568
Eunju Kim: https://orcid.org/0000-0002-7299-4644
Jihyang Lee: https://orcid.org/0000-0001-5038-8419
Jongcheol Son: https://orcid.org/0000-0001-8884-7422
Kyeongyoon Woo: https://orcid.org/0000-0002-5130-8721
Heeyun Noh: https://orcid.org/0000-0001-5301-8675




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download