MATERIALS AND METHODS
Data were retrospectively collected by reviewing all the charts of patients with known metastatic pathologic fractures from 1 January 1995 to 31 December 2013. We identified all patients who underwent operations for pathologic fractures secondary to metastatic tumors during this period in our operating suites. All the medical charts were reviewed until a minimum of two years or death after surgeries. The diagnosis was intra- or postoperatively confirmed in all patients by histopathologic examination. We collected data on age, gender, body mass index, origin of primary tumor, fracture site, visceral metastasis, therapeutic history, surgical time, blood transfusion, net fluid balance, number of postoperative days in the intensive care unit (ICU), complications, and days to death.
We used a variety of severity scores, including the classification of the American Society of Anesthesiologists, Eastern Cooperative Oncology Group performance status (ECOG), Acute Physiology and Chronic Health Evaluation (APACHE) II, Logistic Organ Dysfunction score (LODS), and Charlson score to explore their correlation to the operation-related risk.
The result of surgery was graded as an unsuitable outcome if any of the following three criteria were met: presence of significant complications within 30 days postoperatively which possibly result in serious morbidity, postoperative ICU admission exceeding 2 days, and death from operation for fracture less than 6 months postoperatively. Accordingly, patients were divided into the suitable and unsuitable group. Odds ratio (OR) comparisons included lung, liver, and stomach tumors with the other tumors, femur fractures with other fracture sites, and general, combined general and regional, and regional anesthesia. The cases were analyzed with respect to use of transfusion, intraoperative blood loss volume, operative time, and complications between both groups.
Survival in days was calculated from the day of operation to death. The day of death was determined from medical charts and phone interviews until 31 December 2015. Clinical characteristics were recorded as number (percentage) for categorical variables, and mean ± standard deviation for continuous variables.
Differences between the suitable and unsuitable groups were compared using the Wilcoxon rank sum test for continuous variables and the chi-square or Fisher exact test for categorical variables. Univariable and multivariable logistic regression analyses were performed to identify independent predictors of patients not suitable for surgery. Time to event (180-day death) analysis was performed using Cox proportional hazard regression and reported as hazard ratios (HRs) with 95% confidence intervals (CIs) and as Kaplan-Meier curves with a corresponding log-rank test. We checked proportional hazards assumptions with a test based on Schoenfeld residuals. We selected variables for the adjusted multivariable analysis if their P value was < 0.050 in the univariable analysis. All statistical significance was determined by P < 0.050. All statistical analyses were performed using SAS 9.3 (SAS institute Inc., USA).
Go to :

RESULTS
Forty five patients comprised 18 men and 27 women and age range was from 40 to 96 years. The age distribution was the 8th decade (n = 14), 7th decade (n = 11), 9th decade (n = 10), 6th decade (n = 6), 5th decade (n = 3), and 10th decade (n = 1). The mean body mass index is 21.3. Lung, liver and stomach carcinomas accounted for most of the primary lesions (
Table 1). Primary cancer diagnosis was lung (n = 13), liver (n = 6), and stomach (n = 4). Fractures were most often located in the femur (n = 32) followed by the humerus (n = 6) and radius (n = 4) (
Table 1). All fractures were treated with an internal fixation device or prosthetic implant. Twenty-two patients had 36 additional metastases in other organs, most often the spine (n = 8) followed by rib (n = 4), brain (n = 3), and adrenal gland (n = 3) (
Table 1). Before the time of fracture, 12 patients had received a surgical procedure to treat the primary tumor; 11 patients had received chemotherapy and 7 patients had received irradiation (
Table 1).
Table 1
|
Variable |
Number |
|
Primary cancer diagnosis |
|
|
Lung |
13 |
|
Liver |
6 |
|
Stomach |
4 |
|
MUO*
|
3 |
|
Thyroid |
2 |
|
Breast |
2 |
|
Prostate |
2 |
|
Cervix |
2 |
|
Esophagus |
2 |
|
Colon |
2 |
|
Hematopoiesis |
2 |
|
Others†
|
5 |
|
Sites of pathologic fractures |
|
|
Femur |
32 |
|
Humerus |
6 |
|
Radius |
4 |
|
Spine |
2 |
|
Ilium |
1 |
|
Site of additional metastases to viscera |
|
|
Spine |
8 |
|
Rib |
4 |
|
Humerus |
2 |
|
Other bones‡
|
3 |
|
Brain |
3 |
|
Adrenal gland |
3 |
|
Liver |
2 |
|
Pancreas |
2 |
|
Peritoneum |
2 |
|
Pleura |
2 |
|
Other viscera§
|
5 |
|
Therapy |
|
|
Previous operation on for primary cancer |
12 |
|
Previous operation on for other cancer |
2 |
|
Chemotherapy |
11 |
|
Irradiation |
7 |
|
Others¶
|
5 |

The distribution of American Society of Anesthesiologists physical status classification is 1 (n = 2), 2 (n = 13), 3 (n = 23), and 4 (n = 7). The distribution of ECOG performance status scale is 0 (n = 10), 1 (n = 15), 2 (n = 11), 3 (n = 5), and 4 (n = 4). The mean APACHE II score is 10.5, LODS score 3.42, and Charlson co-morbidity index score 8.9. At review, 30 patients (66.7%) had expired within 2 years postoperatively. Average postoperative survival was 621 days until 31 December 2015. Of the deaths, seven men and seven women had died by 6 months postoperatively. The mean survival time was 355 days for men and 798 days for women. This difference failed to reach statistical significance (P > 0.050). Twenty-eight patients (62.2%) underwent exclusively general anesthesia. Six patients underwent regional anesthesia in addition to general anesthesia. Eleven patients received regional anesthesia only. The mean operating time was 97.3 minutes. Seventeen (37.8%) patients received blood transfusion during surgery. The average net fluid balance for 12 patients in each group exceeded 200 ml.
Twenty patients were admitted to the ICU postoperatively. The mean length of admission was 3.1 days (range, 2–20 days). Two of these patients received mechanical ventilation. Sixteen patients (35.6%) developed postoperative complications including wound infection, bronchopneumonia, pulmonary thromboembolism, pressure sores, and nonunion (
Table 2).
Table 2
Postoperative Complication
|
Variable |
Case |
|
Pneumonia |
4 |
|
Delirium |
3 |
|
Acute renal failure |
2 |
|
Coagulopathy |
1 |
|
Pulmonary thromboembolism |
1 |
|
Pulmonary edema |
1 |
|
Pleural effusion |
1 |
|
Sore |
1 |
|
Wound infection |
1 |

Death within 180 days occurred in 13 patients, admission to ICU beyond 2 days in nine patients, and significant complications in 16 patients. Twenty-two patients collectively were in the unsuitable group. The mean age of the suitable and unsuitable group was 67.1 and 72.3 years, respectively, with no statistical significance.
Table 3 shows the crude and adjusted odds ratios for comparing between suitable and unsuitable groups. Lung tumor was most prevalent and so was chosen as the primary tumor for purposes of comparison. In terms of lung cancer, 2 patients were in the suitable group and 11 in the unsuitable group in 180 days. The proportion of lung cancer differed significantly between the suitable group and unsuitable group (P < 0.050). Lung carcinoma presented a significant OR (P < 0.050) (
Table 4). Among 32 patients with femur fractures, 15 were in the suitable group and 17 in the unsuitable group (
Table 3). The number of additional organ metastases do not have influence on the survival (
Fig. 1), Presence of femur fracture did not have a significant OR (P > 0.050) (
Table 4). Among previous therapy and the scores studied, only the APACHE II score had a statistically significant OR. The APACHE II score was statistically lower in the suitable group compared with the unsuitable group (P < 0.050) (
Table 4).
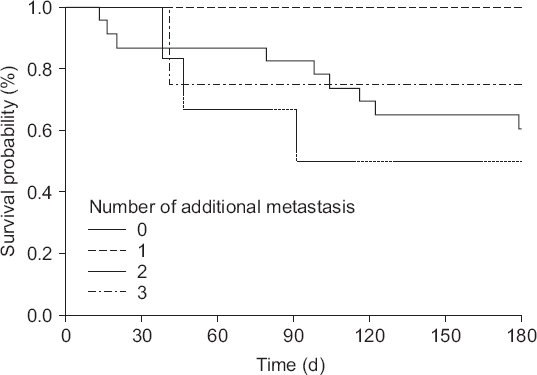 | Fig. 1Kaplan-Meier survival curves according to the number of additional organ metastasis. 
|
Table 3
Association between Patients who are Suitable and Unsuitable for Surgery and Clinicopathological Factors
|
Variable |
Suitable (n = 23) |
Unsuitable* (n = 22) |
P value |
|
Gender, female |
14 (51.9) |
13 (48.2) |
0.903†
|
|
Age (yr) |
67.1 (13.5) |
72.3 (11.5) |
0.242§
|
|
BMI (kg/m2) |
21.5 (3.5) |
21.2 (4.5) |
0.474§
|
|
Tumor |
|
|
|
|
Others cancer |
21 (65.6) |
11 (34.4) |
0.002†
|
|
Lung cancer |
2 (15.4) |
11 (84.6) |
|
|
Additional metastasis |
11 (50.0) |
11 (50.0) |
0.884†
|
|
Fracture site |
|
|
|
|
Other bones |
8 (61.5) |
5 (38.5) |
0.372†
|
|
Femur |
15 (46.9) |
17 (53.1) |
|
|
History of cancer operation |
5 (41.7) |
7 (58.3) |
0.445†
|
|
Chemotherapy |
5 (45.5) |
6 (54.5) |
0.666†
|
|
Irradiation |
3 (42.9) |
4 (57.1) |
0.700‡
|
|
ECOG scale |
|
|
|
|
0 |
6 (60.0) |
4 (40.0) |
0.266‡
|
|
1 |
10 (66.7) |
5 (33.3) |
|
|
2 |
3 (27.3) |
8 (72.7) |
|
|
3 |
3 (60.0) |
2 (40.0) |
|
|
4 |
1 (25.0) |
3 (75.0) |
|
|
ASA PS classification |
|
|
|
|
1 |
1 (50.0) |
1 (50.0) |
0.037‡
|
|
2 |
10 (76.9) |
3 (23.1) |
|
|
3 |
11 (47.8) |
12 (52.2) |
|
|
4 |
1 (14.3) |
6 (85.7) |
|
|
APACHE II score |
8.4 (4.5) |
12.8 (4.7) |
0.003§
|
|
LODS score |
3.1 (1.1) |
3.73 (2.0) |
0.403§
|
|
Charlson score |
8.8 (1.0) |
9.1 (1.2) |
0.712§
|
|
Anesthesia |
|
|
|
|
RA |
3 (27.3) |
8 (72.7) |
0.148‡
|
|
GA |
17 (60.7) |
11 (39.3) |
|
|
GARA |
3 (50.0) |
3 (50.0) |
|
|
Operating time (min) |
94.4 (49.8) |
100.4 (43.6) |
0.322§
|
|
The presence of blood transfusion |
|
|
|
|
No |
15 (53.6) |
13 (46.4) |
0.672†
|
|
Yes |
8 (47.1) |
9 (52.9) |
|
|
Net fluid balance per hour |
|
|
|
|
≤ 200 ml |
11 (52.4) |
10 (47.6) |
0.873†
|
|
> 200 ml |
12 (50.0) |
12 (50.0) |
|

Table 4
Univariable and Multivariable Logistic Regression of Unsuitable Group for Surgery
|
Variable |
Crude OR (95% CI) |
P value |
Adjust OR (95% CI) |
P value |
|
Gender, female |
0.93 (0.28–3.06) |
0.903 |
|
|
|
Age (yr) |
1.04 (0.99–1.09) |
0.903 |
|
|
|
BMI (kg/m2) |
0.98 (0.85–1.14) |
0.792 |
|
|
|
Tumor, lung cancer |
10.50 (1.97–55.97) |
0.006 |
8.39 (1.38–50.90) |
0.021 |
|
Additional metastasis |
1.09 (0.34–3.51) |
0.884 |
|
|
|
Fracture site, femur |
1.81 (0.49–6.76) |
0.375 |
|
|
|
History of cancer operation |
1.68 (0.44–6.39) |
0.447 |
|
|
|
Chemotherapy |
1.35 (0.35–5.28) |
0.666 |
|
|
|
Irradiation |
1.48 (0.29–7.54) |
0.636 |
|
|
|
ECOG scale (reference = 0) |
|
|
|
|
|
1 |
0.75 (0.14–3.94) |
0.734 |
|
|
|
2 |
4.00 (0.64–25.02) |
0.138 |
|
|
|
3 |
1.00 (0.11–8.95) |
> 0.999 |
|
|
|
4 |
4.50 (0.34–60.15) |
0.256 |
|
|
|
ASA PS classification (reference = 1) |
|
|
|
|
|
2 |
0.30 (0.01–6.38) |
0.440 |
|
|
|
3 |
1.09 (0.06–19.63) |
0.953 |
|
|
|
4 |
6.00 (0.18–196.12) |
0.314 |
|
|
|
APACHE II score |
1.24 (1.06–1.45) |
0.007 |
1.22 (1.03–1.44) |
0.024 |
|
LODS score |
1.29 (0.87–1.92) |
0.207 |
|
|
|
Charlson index |
1.26 (0.72–2.22) |
0.415 |
|
|
|
Anesthesia |
|
|
|
|
|
GA |
2.16 (0.52–8.90) |
0.287 |
|
|
|
GARA |
Reference |
|
|
|
|
RA |
1.80 (0.30–10.64) |
0.517 |
|
|
|
Operating time |
1.00 (0.99–1.02) |
0.666 |
|
|
|
The presence of blood transfusion |
1.30 (0.39–4.34) |
0.672 |
|
|
|
Net fluid balance per hour > 200 ml |
1.10 (0.34–3.55) |
0.873 |
|
|

Univariate logistic regression analysis identified lung cancer (OR = 10.50, P = 0.006) and higher APACHE II (OR = 1.24, P = 0.007) as factors associated with unsuitable group (
Table 4). The type of anesthesia, operating time, the need for blood transfusion and net fluid balance did not significantly affect either patient group in terms of OR (
Table 4). Multivariable logistic regression analysis after adjustment, lung cancer (OR = 8.39, P = 0.021) and APACHE II (OR = 1.22, P = 0.024) also remained statistically significant.
In terms of the analysis in comparing between survivors and non-survivors, the median follow-up duration of the survivors was 310 days. Univariable analysis demonstrated significant independent risk factors for 180-day mortality, which included lung cancer (HR = 5.26, 95% CI: 1.71–16.20, P = 0.004) and higher APACHE II score (HR = 1.14, 95% CI: 1.02–1.26, P = 0.022 per 1 point increase) (
Table 5). Kaplan-Meier survival curve shows a significant difference comparing lung cancer to others by log rank test (P < 0.001) (
Fig. 2). Finally, the only statistically significant factor after multivariable analysis was the lung cancer increasing the risk of death (HR = 4.17, 95% CI: 1.16–15.05, P = 0.029) (
Table 5).
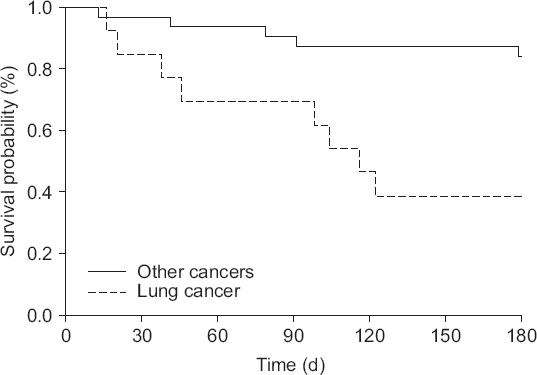 | Fig. 2Kaplan-Meier survival curves comparing between lung cancer and other cancers. 
|
Table 5
Univariable and Multivariable Cox Proportional-Hazards Regression for 180 Days Overall Survival
|
Variable |
Survival (n = 32) |
Death (n = 13) |
Crude HR (95% CI) |
P value |
Adjusted HR (95% CI) |
P value |
|
Gender, female |
21 (77.80) |
6 (22.20) |
2.13 (0.72–6.36) |
0.174 |
|
|
|
Age (yr) |
68.56 (12.87) |
72.31 (12.18) |
1.03 (0.98–1.08) |
0.302 |
|
|
|
BMI (kg/m2) |
21.69 (4.19) |
20.40 (3.46) |
0.94 (0.81–1.09) |
0.392 |
|
|
|
Tumor, lung cancer |
5 (15.63) |
8 (61.54) |
5.26 (1.71–16.20) |
0.004 |
4.17 (1.16–15.05) |
0.029 |
|
Additional metastasis |
18 (56.25) |
4 (30.77) |
0.43 (0.13–1.40) |
0.161 |
|
|
|
Fracture site, femur |
22 (68.75) |
10 (76.92) |
1.45 (0.40–5.26) |
0.575 |
|
|
|
History of cancer operation |
8 (25.00) |
4 (30.77) |
1.32 (0.41–4.29) |
0.643 |
|
|
|
Chemotherapy |
8 (25.00) |
3 (23.08) |
0.98 (0.27–3.55) |
0.972 |
|
|
|
Irradiation |
5 (15.63) |
2 (15.38) |
1.04 (0.23–4.68) |
0.963 |
|
|
|
ECOG scale |
|
|
|
|
|
|
|
0 |
8 (25.00) |
2 (15.38) |
|
|
|
|
|
1 |
10 (31.25) |
5 (38.46) |
1.74 (0.34–8.98) |
0.507 |
|
|
|
2 |
9 (28.13) |
2 (15.38) |
0.90 (0.13–6.37) |
0.913 |
|
|
|
3 |
3 (9.38) |
2 (15.38) |
2.21 (0.31–15.68) |
0.429 |
|
|
|
4 |
2 (6.25) |
2 (15.38) |
3.43 (0.48–24.43) |
0.218 |
|
|
|
ASA PS classification |
|
|
|
|
|
|
|
1 |
2 (6.25) |
0 (0.00) |
- |
- |
|
|
|
2 |
11 (34.38) |
2 (15.38) |
Reference |
|
|
|
|
3 |
16 (50.00) |
7 (53.85) |
2.39 (0.50–11.52) |
0.277 |
|
|
|
4 |
3 (9.38) |
4 (30.77) |
4.74 (0.86–26.03) |
0.073 |
|
|
|
APACHE II score |
9.44 (5.28) |
13.15 (3.34) |
1.14 (1.02–1.26) |
0.022 |
1.08 (0.96–1.22) |
0.222 |
|
LODS score |
3.28 (1.46) |
3.77 (1.83) |
1.23 (0.87–1.72) |
0.242 |
|
|
|
Charlson index |
8.84 (0.95) |
9.08 (1.38) |
1.33 (0.74–2.40) |
0.339 |
|
|
|
Anesthesia |
|
|
|
|
|
|
|
RA |
5 (15.63) |
6 (46.15) |
3.42 (1.10–10.66) |
0.034 |
2.82 (0.87–9.07) |
0.083 |
|
GA |
22 (68.75) |
6 (46.15) |
Reference |
|
Reference |
|
|
GARA |
5 (15.63) |
1 (7.69) |
0.80 (0.10–6.66) |
0.838 |
1.79 (0.19–17.36) |
0.615 |
|
Operation time |
92.75 (46.50) |
108.62 (46.19) |
1.01 (1.00–1.02) |
0.351 |
|
|
|
The presence of blood transfusion |
11 (34.38) |
6 (46.15) |
1.27 (0.43–3.80) |
0.663 |
|
|
|
Net fluid balance per hour > 200 ml |
18 (56.25) |
6 (46.15) |
0.77 (0.26–2.29) |
0.635 |
|
|

Go to :

DISCUSSION
The incidence of metastatic pathologic fracture has increased because of the longer survival of cancer patients [
7]. Skeletal metastases are a common occurrence in up to 80% of cancer patients [
7]. Bone is a common site of metastases, after liver and lungs [
10]. Pathologic fractures occur in 8% to 30% of patients with bone metastases [
7,
10,
11]. Once a fracture occurs, the terminally ill cancer patient is at an increased risk for a variety of complications that can hasten death [
3]. The risk of death associated with a pathologic fracture is increased by about 20% in these patients [
3]. Treatment of pathologic fractures is by surgical stabilization, if possible [
7,
12]. The goal of palliative surgery is to lengthen life span, restore stability, preserve ambulation, alleviate severe pain that is unresponsive even to narcotics, and ultimately to optimize the quality of life [
2,
6,
8,
13]. However, the absolute indication and timing for palliative surgery for pathologic fractures is controversial [
13,
14].
Tumors arising from the lung, breast, prostate, colon, thyroid, and kidney as well as multiple myeloma are prone to spread to bone [
3,
9]. Our case series is similar with other reports, where the most frequent primary tumor is lung tumor [
4]. Patients with metastatic lung cancer have the worst prognosis as it is a highly aggressive neoplasm [
5,
13,
15]. Our relatively high incidence of liver and stomach cases might be the reflection of the high prevailing incidence of these cancers in Korea [
16].
The femur is the most frequent affected site of fracture because of metastatic cancer. Other sites that are frequently affected by bone metastases include the vertebral column, ribs, pelvis, radius, and skull [
2,
6,
8,
10]. The short survival period after pathologic fracture of the long bones has been reported in other study [
17]. However, our finding about mortality in the patients with femur fractures is observed to be insignificant.
Surgical treatment of pathologic fractures may be followed soon by death, contrary to expectations [
2]. Surgery is not advised if a patient is too debilitated to withstand the surgery, or if the expected survival time is too short to recover sufficiently to benefit from the operation [
8]. Palliative operations that are performed indiscreetly could be devastating for the patients [
3]. Serious complication or unexpected early death would compound the unnecessary suffering including apprehension for the operation and postoperative pain. Some patients with a limited life expectancy may undergo unnecessary palliative operations [
8]. We hypothesized that these poor clinical results, with some immediate postoperative deaths in these very ill patients, suggest the patient of surgery can be selected inappropriately. If it is anticipated that patients will require intensive care facilities and experience significant postoperative complications in terms of their surgery-related condition, then palliative surgery is unjustified [
13]. We modified these suggestions to apply to our hypothetical conditions.
There is little agreement on allowance of remaining lifespan on which time limit to base the decision of operation [
17]. Patients with a life expectancy of less than six weeks rarely gain useful benefit from major surgery [
13,
14]. In present study, most of the reports recommended the remaining survival as 6 months for the palliative operation [
13,
18].
Predicting life expectancy in terminal cancer is important for clinicians because a poor survival estimate can make a clinical decision about appropriate palliative treatment harder [
19]. In general, lifespan is difficult to estimate accurately [
14,
19]. If there are the clinical condition (the common terminal pathway) that include precipitously decreased performance, anorexia-cachexia, dyspnea, dysphagia, and delirium, the signs should preclude the administration of general anesthesia [
7,
14,
19].
Several disease severity and organ dysfunction scoring systems including American Society of Anesthesiologists physical status classification [
15], ECOG scale, which is the scoring system for performance status in terminal cancer patients [
20], APACHE II score [
21], LOD system score [
22], and Charlson co-morbidity index [
23,
24], which could reflect numerous comorbid conditions, were presently applied to discern their predictable capability. We suggested the possibility of APACHE II scoring in making clinical decision of surgery for patients with pathologic fractures.
The underlying diseases in cancer patients include malnutrition, superimposed infection, chemotherapy-induced lung injury, ectopic hormone production and electrolyte imbalance including hyponatremia, or hypercalcemia as well as local effects of cancers and distant metastases [
9,
15,
25]. The affected patients are mostly older, at an advanced stage, and have medically incurable diseases including rejected chemotherapy by their own will and recognition of the potential adverse effects of opioids in some narcotic-dependent patients [
15]. It is essential that the general poor condition of the patient is addressed before surgery, if possible [
13,
15]. However, it is often impossible, as in performing breathing exercises to optimize pulmonary function and hyperalimentation to correct nutritional deficiencies [
15]. In addition, multiple sites of bony metastases and the presence of visceral metastases are associated with a poor prognosis [
7,
9,
12]. Additional organ involvements lower survival compared to patients whose disease remains clinically confined to the skeleton [
9]. However, the mean postoperative survival for patients with metastases confined to the operated bone compared to patients with coexistence of osseous and nonosseous metatstases was not statistically significance according to the number of organs and other bones in our study due to unknown reason. Reportedly, patients with more co-morbidities, uncontrolled primaries, and widespread metastases experience heightened mortality [
26].
Postoperative cell-mediated immunity suppression, the first-line defense mechanism against cancer, is associated with underlying illness, direct effect of anesthetics, pain, stress-related hormonal changes, hemorrhage and transfusions, ischemia-reperfusion, and factors including age and gender [
27]. Therefore, we investigated to find out more about these effects on our patients.
Gottschalk et al. [
27] suggest the hypothesis that surgical stress response increases the likelihood of cancer dissemination and metastasis during and after cancer surgery. Anesthetic method in cancer patient might potentially influence outcome including long-term survival [
25]. Therefore the possible method for metastasis prevention by the anesthesiologist opioids, COX inhibitor, α
2-adrenergic agonist, β-adrenergic blockade, regional anesthesia, suppression of blood transfusion, and perioperative hypothermia was suggested [
27]. The type of anesthesia, especially regional anesthesia, may play a role in this process and could indirectly affect malignant cell development and attenuate surgery-induced increases in malignant proliferation [
27]. The addition of regional anesthesia to general anesthesia also results in less overall use of volatile anesthetics and opioids, theoretically resulting in less immunosuppression [
27]. Further studies are needed to establish the benefits of regional anesthesia in patients with pathologic fractures given in our result.
Cancer patients who receive blood transfusions during surgery may tend to do worse probably due to transfusion-related immunosuppression [
27]. The present findings do indicate that this is unlikely. It may be that both anemia and blood transfusions are associated with harmful effects in cancer patients. Perhaps factors influencing the need for blood transfusion have a greater influence on prognosis than the receipt of blood itself [
27]. The duration of anesthesia and net fluid balance also have not been associated with an increased risk for developing morbidity and mortality, both presently and previously study [
27].
The intraoperative occurrence of difficult ventilation, intubation, and subsequent hypoxemia due to head and neck cancers, history of radiation exposure, pulmonary mass itself, or fat embolism, may be the possible events in these patients [
15,
18,
28]. Pathologic fractures of the ribs also can impair ventilation [
9,
15]. Postoperative complications include pneumonia, respiratory failure, renal failure, disseminated intravascular coagulation, hepatic failure, cerebral infarct, adrenal crisis, vegetative state, and death [
15,
18,
28,
29]. Postoperatively, some patients may require mechanical ventilation or vasopressor therapy for shock [
25].
The present study has several limitations. It was conducted at a single center with a limited sample size. The data of some palliative operations may be outdated. A few decades ago the survival following recognition of bone metastases was poorer than is at present [
30]. Patient variation in preoperative history and antitumor therapy prior to surgery was marked and their preoperative status could not be analyzed in a meaningful way. Heterogeneities concerning the type and complexity of the operations may affect the comparison between the groups. The variety of devices used by the different surgeons does not allow the clear discernment of the effect of the surgical procedure on patient outcome [
2]. Lack of surgeon’s experience in palliative surgery may increase the risk of an undesirable outcome [
28]. We excluded the effects of cell type, stage, and the patient’s detailed health problems because these aspects go beyond the objective of the study. Because of these limitations, our findings must be recognized as preliminary.
In the absence of firm data, the argument for palliative surgery is whether to perform an operation for patients who will realize benefits, or avoid operation for inappropriate patients to eliminate the considerable risks of surgery. Our results provide another warning against reckless application of surgery in these patients. In the absence of reliable indicators of the patient’s life expectancy, the value of these palliative operations cannot help but remain obscure. Anesthesiologists involved in both surgical operations, intensive care, and palliative pain therapy should play a leading role in preoperatively evaluating general status and participate in deciding the plan of palliative surgery within the context of a multidisciplinary team approach.
Palliative surgery for pathologic fracture could likely result in suitable outcome if the patient with high APACHE II score is paid more attention. Our result of lung tumor as main negative factor affecting postoperative survival suggests that a prudent anesthetic approach is essential in patients with lung carcinoma. We conclude that a patient’s preoperative medical condition is the major determining factor for the postoperative outcome. Factors associated with anesthesia themselves do not have a statistically meaningful correlation with the prognosis of these patients. Clearly more time and resources are required to refine the safe anesthetic management in terminal cancer patients with pathologic fractures.
Go to :





 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download