INTRODUCTION
Interscalene brachial plexus block (ISB) is one of the most effective methods for anesthesia and postoperative analgesia after shoulder arthroscopic surgery [
1–
8]. Ultrasound guidance, compared with anatomical landmark and paresthesia techniques, can provide direct visualization of the target nerve, surrounding tissue, and injectate spread, and may lead to improvement in patient safety for decreased nerve injury or other serious complications including local anesthetic systemic toxicity and pneumothorax [
5,
7,
8–
11]. In several literatures, the use of ultrasound significantly enables low volumes of brachial plexus block to reduce the incidence and intensity of hemidiaphragmatic paresis (HDP), but has still no significant effect on the incidence of postoperative neurologic symptoms (PONS) [
12,
13]. Many patients and surgeons tend to choose general anesthesia instead of a block or a block combination with general anesthesia, because they lack understanding of the possible complications and because of anxiety related to inserting a needle in the neck during ISB. In particular, the most common misconception is that a nerve block is associated with an increased risk of nerve injury [
14].
The patient, surgical technique, position, and ISB-related risk factors can contribute to PONS after an arthroscopic shoulder operation. Technical skills, such as needle manipulation during ISB using a nerve stimulator, as well as anatomical landmarks and the types of local anesthetic drug used for ISB, are important neurological risk factors [
1]. However, use of ultrasound (US) has enabled safe and effective ISB, so anesthesiologists need to differentiate between these factors when patients develop PONS [
5,
7,
8,
9–
11]. PONS can be acute, long-term, or delayed depending on the time of expression [
1,
4]. These include not only transient and reversible problems, such as paresthesia, dysesthesia, and pain not clearly related (or unrelated) to surgery, but also permanent severe neuropathy, motor deficits, and muscular weakness. In rare cases, major and fatal complications, such as seizure and cardiovascular collapse, have been reported [
1–
4].
We retrospectively evaluated and reviewed US-ISB related safety in the form of HDP and PONS in patients undergoing arthroscopic shoulder surgery.
Go to :

MATERIALS AND METHODS
This was a retrospective analysis of all shoulder patients who received an ISB at our hospital from January 2010 to May 2015 after obtaining the Institutional Review Board approval (EUMC 2015-08-028). The patient medical records were reviewed for demographic information, American Society of Anesthesiologists physical status classification, underlying diseases, operative time, anesthetic time, type of surgical procedure, local anesthetics used for ISB, and postoperative complications. Shoulder operations were performed on 1,258 patients; 311 patients underwent open reduction and internal fixation, 69 underwent total shoulder replacement, 13 underwent debridement and biopsy of the shoulder joint, and 11 underwent other procedures. In total, 854 patients were enrolled in our study, of whom 182 were excluded because they did not receive an ISB, and 4 were excluded because of conversion to open surgery. Therefore, 668 patients were analyzed in this study (
Fig. 1). Operations were performed under general anesthesia. Patients who had PONS after ISB were checked for a motor deficit (weakness) or sensory deficit (loss of feeling), such as hypoesthesia, defined as numbness; paresthesia, defined as an abnormal but not unpleasant sensation; and pain dysesthesia, defined as an unpleasant abnormal sensation [
1]. The patients were assessed at 48 hours, 2 weeks, 1 month, 3 months, and 6 months after operation. In some cases, patients were followed up until the symptoms improved. We determined whether these PONS were associated with ISB or surgery.
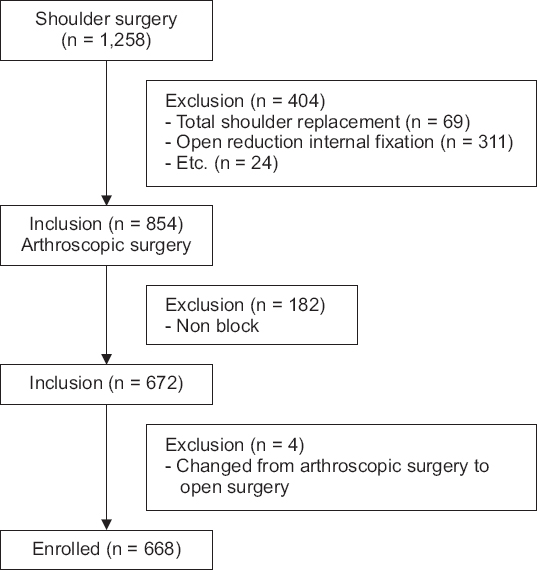 | Fig. 1Flow chart of the study design. 
|
Statistical analyses were performed using IBM SPSS software ver. 18.0 (IBM Corp., USA). Descriptive data are presented as numbers (%) or mean ± standard deviation. A P value < 0.05 was considered significant.
Our standard management for patients undergoing shoulder surgery was as follows.
Block techniques
Following general screening for the operation, the patients were scheduled for surgery at a preoperative anesthetic visit before admission, provided they were of American Society of Anesthesiologists physical status classification 1–3, had no contraindications to ISB, and agreed to follow the written instructions provided to them regarding anesthesia and pain control. Absolute exclusion criteria included patient refusal, presence of coagulopathy, infection at the block site, any neurological deficit in the surgical limb, severe lung disease, contralateral diaphragmatic paralysis, or known allergy to local anesthetics.
Standard American Society of Anesthesiologists monitors were used throughout the surgery. All patients were taken single shot ISB under US guidance (M-Turb, SonoSite Inc., USA). The blocks were performed by one of two experienced anesthesiologists or a supervisor, and were facilitated by sedation using 1–3 mg midazolam and 25–50 μg fentanyl intravenously. The patient was placed in a supine position with the head turned away from the side to be blocked. Using a high frequency (5–12 MHz) linear probe, the hypoechoic nerve roots or the superior trunk (between the anterior and middle scalene muscles) was identified in the short-axis view. After sterilizing the skin, the point where the C5, C6, and C7 roots or superior trunk were most visible was selected. A 50 mm, 22-gauge insulated needle (Stimuplex A, B.Braun, Germany) was used, and the needle tip was advanced between the C5 and C6 roots or superior trunk within the sheath using the in-plane method. After localization and negative aspiration, attempts were made for each US-guided block to create a halo or local anesthetic application to visualize the spread and confirm nerve sheath. After the US-ISB, general anesthesia was induced using 4 mg/kg pentothal sodium or 1–2 mg/kg propofol and 1–2 mg/kg fentanyl. Tracheal intubation was facilitated with 0.6 mg/kg rocuronium. Anesthesia was maintained using a 50% oxygen/air or N2O mixture and sevoflurane or desflurane inhalation anesthetics (1.0–1.5-fold the minimum alveolar concentration) to maintain the bispectral index value at 40–60 and systolic blood pressure within 20% of the baseline value. Patients were placed in the lateral decubitus position. All surgeries were performed by a single surgeon. Patients were extubated in the operating room after reversing any residual muscle relaxation.
In the recovery room, patients were assessed by an anesthesiologist using a numerical rating scale (NRS) for pain, arm weakness, arm numbness, occurrence of possible complications including HDP. All patients underwent plain chest radiography as well as surgical postoperative radiographic evaluations.
Pain control
Postoperative pain was evaluated using the NRS (0, no pain; 10, most severe pain imaginable) up to 48 hours from the time of arrival in the recovery room, proceeded by an anesthesiologist. The patients were administered 100 mg intravenous tramadol (up to 300 mg/day) when the NRS was > 4 or the patient requested analgesia with or without intravenous patient-controlled analgesia. Patients were treated with 30 mg intravenous ketorolac (up to 90 mg/day) in cases of insufficient analgesia by an orthopedic surgeon.
After discharge, patients were prescribed an oral nonsteroidal anti-inflammatory drug (NSAID, Celecoxib 200 mg) once daily for 2 weeks along with a standard rehabilitation program. In addition, a US-guided subacromial corticosteroid injection, consisting of 40 mg triamcinolone and 4 ml of 2% lidocaine, was administered to patients who woke up at night because of severe shoulder pain, or whose pain was exacerbated at the time of rehabilitation within 8 weeks after the operation, and they were provided with an additional 2-week NSAID prescription by an orthopedic surgeon.
Go to :

RESULTS
The demographic and surgical data are summarized in
Table 1. The mean volume of local anesthetic used was 11.5 ml, including levobupivacaine (0.5%, 28%) and ropivacaine (0.5%–0.75%, 72%), with 5 mg dexamethasone added in 77.6% of cases. The surgical procedure types were as follows: 417 of 668 (62.4%) cases had a rotator cuff tear repair and 122 of 668 (18.3%) had a Bankart repair. Mean anesthetic time was 131.5 minutes, and mean operation time was 74.9 minutes.
Table 1
Demographic and Clinical Data
|
Variable |
Value |
|
Age |
52.6 ± 16.4 |
|
Sex (M/F) |
428/240 |
|
BMI (kg/m2) |
24.6 ± 3.3 |
|
ASA PS (1/2/3) |
280/385/3 |
|
Underlying disease |
|
|
Hypertension/DM |
130/26 |
|
COPD/Asthma |
1/15 |
|
Local anesthetics |
|
|
0.5% levobupivacaine |
59 (8.8) |
|
0.5% levobupivacaine + 5 mg dexamethasone |
128 (19.2) |
|
0.75% ropivacaine |
91 (13.6) |
|
0.5% ropivacaine + 5 mg dexamethasone |
390 (58.4) |
|
Volume of local anesthetics (ml) |
11.5 ± 0.3 |
|
Anesthetic time (min) |
131.5 ± 36.4 |
|
Operative time (min) |
74.9 ± 32.7 |
|
Surgical procedure type |
|
|
Rotator cuff tear repair |
417 (62.4) |
|
SLAP repair |
39 (5.8) |
|
Bankart repair |
122 (18.3) |
|
Capsular release for frozen shoulder |
17 (2.5) |
|
Subacromial decompression and calcification |
20 (3.0) |
|
Other |
53 (7.9) |

Three patients (0.4%) complained of mild tightness after entry into the recovery room, but an arterial blood gas analysis (ABGA) was normal. HDP was detected on chest radiograph in postanesthetic care units. All of these patients improved immediately within 1 day after surgery.
Thirty-two patients (4.8%) developed PONS; seventeen patients (1.8%) had symptomatic relief within 2 weeks, eleven (1.5%) improved after 3 months, and four (0.4%) improved after 6 months. No permanent neuropathy was observed (
Fig. 2). Two cases (0.1%) developed PONS likely associated with US-ISB; one case developed paresthesia (tingling sensation) in the thumb and index finger 2 days after the operation. The patient refused consultation, and symptoms improved by 14 days postoperatively. The other case developed persistent left posterior auricular nerve area hypoesthesia 2 days after surgery. The ear-nose-throat and neurology departments were consulted but the symptoms progressed after 6 months of follow-up without further examination or treatment. PONS were not likely associated with US-ISB in thirty cases (4.5%); two (0.3%) patients developed hypersensitivity in the supraclavicular and axillary nerve dermatome areas, which improved after 2 weeks and 3 months, respectively; twenty-six patients (4.3%) complained of dysesthesia (pain) in the supraclavicular and axillary nerve dermatome area, and 15 improved within 2 weeks after drug treatment. Eleven patients (1.6%) received an additional intra-articular steroid injection due to pain insomnia and continuous, severe pain (> 7 points on the NRS); ten of these patients improved within 3 months, but the other case underwent a re-operation due to a rotator cuff tear relapse 6 months after the first surgery. Two patients (0.3%) complained of pain and dysesthesia with motor weakness; one received an intra-articular steroid injection but underwent a reoperation due to avascular necrosis of the humeral head 4 months after surgery. In another case, the pain decreased after a steroid injection administered 3 months after surgery, and the weakness recovered after 6 months of follow-up (
Table 2). PONS that occurred after the shoulder arthroscopic operation were confirmed to improve within 6 months but two patients (0.3%) needed a re-operation for PONS not likely associated with US-ISB.
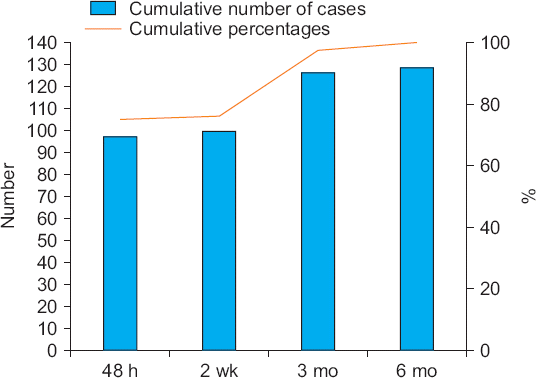 | Fig. 2The incidence of resolved neurological complications over time after arthroscopic shoulder surgery under general anesthesia combined with ultrasound guided-interscalene brachial plexus block. wk: week, mo: month. 
|
Table 2
Details of Postoperative Neurological Symptoms of Arthroscopic Shoulder Surgery under General Anesthesia Combined with Ultrasound Guided-interscalene Brachial Plexus Block (US-ISB)
|
Adverse event |
Incidence |
Description |
Resolution |
|
Neurologic symptoms likely association with the US-ISB |
|
Hypesthesia |
1 (0.1) |
Numbness in the posterior auricular nerve area |
Improved spontaneously within 6 months |
|
Paresthesia |
1 (0.1) |
Paresthesia on the tip of thumb/index finger |
Improved spontaneously within 2 weeks |
|
Neurologic symptoms not likely association with the US-ISB |
|
Hypesthesia |
2 (0.3) |
Supraclavicular/axillary nerve area |
Improved spontaneously within 14 days, 3 months respectively |
|
Pain dysesthesia |
26 (4.3) |
Supraclavicular/axillary nerve area: observation (15)/further treatment (11) |
Resolution < 3 months (15), Steroid injection (10)
Reoperation (1) due to re-tear → resolution |
|
Pain with motor weakness |
2 (0.3) |
One case of avascular necrosis of humeral head shoulder confirmed despite medical treatment; the other resolved within 6 months with observation |
Total shoulder replacement performed after 4 months later → resolution |

Go to :

DISCUSSION
Our retrospective study shows that PONS occurred in 32 (4.8%) of 668 patients who underwent arthroscopic shoulder surgery under general anesthesia combined with US-ISB. Only two cases (0.3%) were likely associated with ISB, and symptoms improved naturally without special treatment within 6 months. No chronic or permanent complications were observed. Many studies have reported nerve injuries that developed after shoulder arthroscopy, with an overall incidence of 10% for transient paresthesia and/or true nerve palsy [
1–
4,
15–
18]. Developed PONS after arthroscopic shoulder surgery with ISB may be mistakenly associated for block-related complications. Selander et al. [
19] indicated that peripheral nerve complications are not directly attributable to performance of the block itself, but that other factors may be responsible. The incidence of peripheral neuropathies observed in the literature after a peripheral nerve block varies from less than 1% to more than 5% [
19]. Borgeat et al. [
1] reported that the incidence of persistent paresthesia, dysesthesia, or pain not related to surgery was 7.9% at 1 month, 3.9% at 3 months, 0.9% at 6 months, and 0.2% at 9 months at the time of ISB via a nerve stimulator and a perineural catheter. Candido et al. [
2] observed an incidence rate of 4.4% for PONS related to the ISB procedure; nine cases (1.3%) developed side effects at the ISB site, such as the phalanx of the thumb/index finger, and seven (1%) developed side effects related to the posterior auricular nerve, based on symptom distribution, all of which stopped after 2–12 weeks. Our incidence of 0.3% for PONS was lower than that reported in these previous ISB studies. Some methodological differences may explain the discrepancies among studies. First, in our institute, the techniques and local anesthetic drugs administered are standardized. The nerve and surrounding tissues were directly identified by US. The proximity of the needle tip to the nerve was maintained, and local anesthetic spread was determined in real time so that intraneural injection was not observed under US guidance [
5,
7,
8–
11]. The ISB procedures were conducted either directly or under the supervision of two highly skilled professionals and the surgical and postoperative pain and rehabilitation programs were also standardized to reduce neurological damage. In addition, 40–50 ml of local anesthetic was used for surgical anesthesia in previous studies [
1–
3]. However, in this study, injecting an average of 11.5 ml of 0.5%–0.75% levobupivacaine and ropivacaine for postop analgesia lowered the incidence of side effects due to the lower volume than previous studies [
5,
6]. Ropivacaine and bupivacaine have a similar duration of action and were suitable for pain relief, with no difference in the frequency of side effects [
20,
21]. In the present study, 5 mg dexamethasone was added to a low-volume (11.5 ml) local anesthetic in 77.5% of cases. Webb et al. [
22] and Woo et al. [
23] have shown that adding a 5 mg steroid prolongs postoperative analgesic duration without neurotoxicity [
23,
24].
Three patients (0.4%) developed HDP, all of whom complained of mild tightness but showed normal ranges on the ABGA. Symptoms improved within 1 day, encouraging them to practice deep breathing after receiving 2 L/min of O
2 insufflation via nasal prong surgery. Rains et al. [
16] reported that a modest degree of phrenic verve block occurs with virtual interscalene block, but transient complications were not a concern unless the patient had severe respiratory compromise or coincident contralateral phrenic paralysis [
24]. Rajpal et al. [
5] reported that lower volumes of local anesthetic may be important for reducing PONS.
Transient HDP is a common side effect on non US-ISB that typically use 20 ml or more local anesthetic. The advent US-ISB not only facilitates more accurate deposition of local anesthetics but lowers the volumes of anesthetics [
25,
26]. Three studies of low volume local anesthetic that aimed to reduce or eliminated HDP by limiting local anesthetics spread to the phrenic nerve during ISB or supraclavicular approach were reviewed. Riazi et al. [
6] and Lee et al. [
21] reported that 5 ml or 10 ml ropivacaine 0.75% produced equal analgesia, but that 5 ml reduced chest X-ray-diagnosed HDP from 60% to 33%. Neal [
27] reported the same incidence of HDP using 10 ml or 20 ml ropivacaine 0.5% was deposited at the cricoid cartilage. However, these desirable effects are not predictable for an individual patient, which could be problematic for those patients who could most benefit from eliminating HDP, that is, those with severe pulmonary disease who require oxygen or long term steroid therapy. US-ISB reduces but does not eliminate the incidence and intensity of HDP to zero [
13].
Twenty-six of our patients had diabetes, and none showed neurological side effects. Boulton et al. [
28] reported that diabetic neuropathy is heterogenous, including generalized symmetric polyneuropathies and focal multifocal neuropathy (cranial, truncal, focal limb, proximal motor and chronic imflammatory demyelinating neuropathy). Patients with diabetic neuropathy may be less responsive to neurostimulation and are vulnerable to ischemic injury and nerve damage after a peripheral nerve block; numbness, pain and autonomic dysfunction also normally occur. All of our patients had controlled diabetes mellitus without neuropathy and the frequency of neurological complications was none that we are unable discuss the relationship between diabetes and neurological complications. Careful selection in diabetic patients and monitoring are necessary before performing ISB. One patient with chronic obstructive pulmonary disease (COPD), who had no symptoms at the incidental preoperative evaluation detection, did not show any respiratory symptoms after ISB. Riazi et al. [
6] reported that phrenic nerve blocks occurred in 45% of patients who received 5 ml and 100% of patients who received 20 ml during US-ISB. In patients with underlying lung disease (such as COPD), and reduced reserve power due to morbid obesity, or of an older age, phrenic nerve block can reduce forced vital capacity by 21%–34%, forced expiratory volume in 1 sec by 17%–37%, peak expiratory flow rate by 15.4%, and lung volume (by a significant amount). The risk of postoperative respiratory complications can increase even further, so it is also necessary to carefully select patients.
Two patients (0.3%) developed ISB-related PONS in our study; one patient (0.1%) had paresthesia confined to the distal phalanx of the thumb and index finger. Plexopathy can occur when the brachial plexus root is injured. Because the needle entry point for ISB, and the site of drug injection, are between the C5 and C6 roots or the superior trunk within the sheath, we presumed that this neurological symptom could be an ISB-related complication. The incidence of this type of complication was lower than that (1.3%) in a previous study and this complication improved within 2 weeks [
2].
The other PONS was numbness in the posterior auricular nerve area, which was likely associated with ISB. Candido et al. [
2] reported six cases of hypoesthesia and one case of dysesthesia on the posterior auricular nerve after ISB. As the great auricular nerve and the cervical cutaneous branches are very close to the needle entry site for ISB, direct trauma caused by the needle can occur. Our case improved after 6 months. In contrast, Park and Kim [
29] reported three cases of neuropraxia of the occipital nerve and auricular nerve after shoulder arthroscopy without ISB. The numbness on the posterior auricular nerve improved recover within 3 weeks after surgery and had resolved completely within 2 months. They speculated that the development and severity of neuropraxia of the superficial branches of the cervical plexus were related to the degree of rotation and deviation of the head and neck, the duration of the procedure, or the direct compression required to fix the head. We carefully determined whether our case of numbness on the posterior auricular nerve was related to either of these two factors or both. Although ISB was performed using the inter-sternocleidomastoid approach according to our standardized protocol, the cervical plexus is not easily visualized using us and the needle may have passed immediately adjacent to the superficial cervical plexus, so that direct needle trauma is highly possible [
29]. We speculate that the neuropraxia occurred due to patient positioning, which was lateral decubitus, with excessive rotation and direct compression while fixing the head and neck [
16–
18]. Because of the combined effects of these two factors, 6 months was needed to recover, which was longer than in previous reports.
Thirty patients (4.5%) developed PONS not likely association with the US-ISB. Several factors, such as patient susceptibility to nerve injury, a particularly subclinical neuropathy, male sex, elbow flexion, and tissue edema play a major role in these symptoms [
2]. In this study, we suspect that surgical factors had more of an influence than patient factors because patients at risk were excluded by our preoperative patient selection procedure. Traction injuries resulting from lateral decubitus positioning, and neurovascular injuries related to arthroscopic portal placement, are responsible for surgical shoulder arthroscopic nerve injuries [
15–
18]. Nerve injuries occur with an incidence of 0.1%–10%, including temporary paresthesia (10%) permanent neurapraxia (2.5%), and musculocutaneous nerve injury at 5 o’clock (rare), and are generally known to recover within a short period of time [
15,
16,
30]. Direct axillary nerve injury due to a lateral port is the main injury type and reportedly occurs in up to 10% of cases; however, most improve within 48 hours. Damage to the supraclavicular nerve can also be caused by anchor pressing, but this causes chronic side effects in < 0.1% of cases. In our retrospective study, twenty-eight (4.2%) cases developed hypoesthesia or pain dysesthesia in the axillary nerve or supraclavicular nerve; most resolved after 3 months, and there was no persistent neurological sequelae [
15].
Our study had several limitations. First, this was a retrospective small sampled study in single center and large and multi-center samples have not been included. Also, there may be more side effects than we actually know if the patients follow-up were stopped or lost because it was a retrospective study. Second, although more experienced anesthesiologists tend to have lower complication rates, we were unable to determine the relationship between anesthesiologist experience or other ISB techniques and complication risk. Third, neurological side effects were difficult to associate with the ISB procedure because of inconsistency in the underlying disease, and in the type and severity of surgery, and because of the relatively small sample size. Fourth, it was not possible to clearly identify the cause of neurological complications arising from the ISB procedure because further diagnostic tests, such as electromyography and magnetic resonance imaging, were not performed because of patients’ refusal, and the symptoms gradually resolved with time.
In conclusion, our retrospective study demonstrated that US-ISB for arthroscopic shoulder surgery is associated with a 0.4% incidence HDP and a 0.3% incidence of PONS related to transient minor sensory symptoms, which spontaneously improved within 6 months. Therefore, we confirm that the US-ISB procedure with low volumes of local anesthetics is a well-accepted technique with a low rate of HDP and PONS.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download