Abstract
Although spinal anesthesia is one of the most reliable anesthetic techniques in clinical practice, failures may occur in daily practice at rare occasions. Their causes are diverse and they include anatomical structural variations. In particular, postoperative anatomical changes often occur in patients who have undergone spine surgery and may cause failures of spinal anesthesia. Postoperative pseudomeningocele constitutes extradural cerebrospinal fluid collected from a dural tear and it is considered a very rare complication of spine surgery. We describe the case where a patient with unexpected postoperative iatrogenic pseudomeningocele received lower extremity surgery under spinal anesthesia.
Spinal anesthesia was introduced more than 100 years ago and is one of the most reliable regional anesthetic methods. Although rare, spinal block failures do occur. Sometimes, dry tap and inadequate block are experienced and the their causes can be leakages of injectates, failed dura punctures, and the displacements of needle tips, in addition to spinal anatomical abnormalities, such as scoliosis, kyphosis, and dural sac abnormalities.
The condition of pseudomeningocele involves extradural cerebrospinal fluid (CSF) collected from a dural tear and can be categorized into following: congenital, traumatic, and iatrogenic [1–4]. The latter mostly occurs after durotomy during spine surgery. Because the size of pseudomeningocele slowly increases, it is generally asymptomatic and most patients generally neglect a cyst’s presence. In the absence of pain, the presence of pseudomeningocele appears not severe to them. Thus, both the patient and the surgeon may forget to consult anesthesiologists about the presence of pseudomeningocele prior to spinal anesthesia. This can confuse anesthesiologists and may be an obstacle to spinal anesthesia.
We present the case of a patient with unexpected postoperative iatrogenic pseudomeningocele, who received lower extremity surgery under spinal anesthesia.
A 67-year-old, 148 cm, 75 kg female was admitted for right total knee arthroplasty. She was diagnosed with diabetes mellitus 10 years ago, and hypertension five years ago. The patient had received lumbar spine surgery three times during six years, most recently one year ago with a L2-5 posterior lumbar interbody fusion. She did not complain about back pain or radiating pain. Preoperative laboratory values including coagulation parameters were within normal ranges at the time of admission.
During the preoperative visit, the patient asked for spinal anesthesia rather than general anesthesia, since she had a past negative general anesthesia experience and we therefore decided in favor of this procedure.
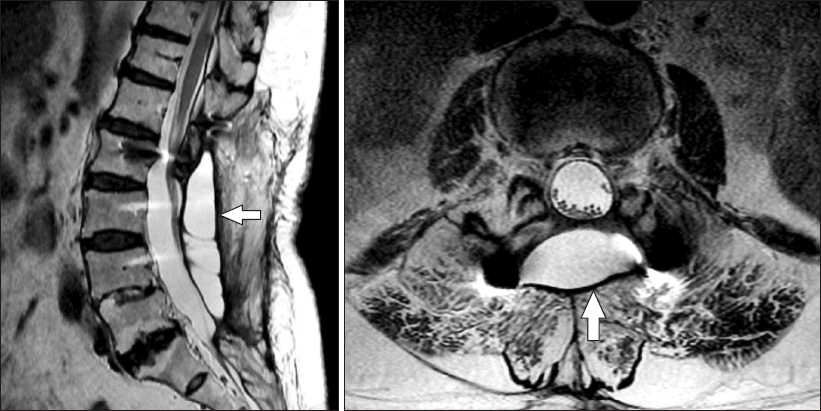
As she came into the operating room, spinal anesthesia was performed in the right lateral decubitus position at the L3-4 level via the midline approach technique with a 25-gauge Quincke spinal needle at the first attempt. Hyperbaric 0.5% bupivacaine 10 mg with fentanyl 10 μg was injected after the subarachnoid space was confirmed by free flow and aspiration of CSF through a needle. After five minutes, with the patient in the right lateral position, the level of sensory block was tested with alcohol swabs, which proved to be unsuccessful. She was turned to the supine position and we waited for 10 minutes with the patient in a head-down position. Even then, the sensory block level could not be achieved. When we explained the failed spinal block situation, the patient told us about a cyst after a prior spine surgery. We reviewed lumbar spine magnetic resonance imaging (MRI) and detected the cyst. MRI displayed a cystic lesion (90 mm × 34 mm × 18 mm) at the L2-5 level (Fig. 1). The cyst fluid had the same signal intensity as the CSF. We thought that the local anesthetic had been injected in the cyst, that this was the reason for the spinal anesthesia failure, and then told the patient that anesthesia had to be reattempted. However, she still insisted on the spinal anesthesia, so we decided to try it again. The distance from the skin to the true dura-arachnoid membrane and the cyst’s A-P diameter at the L3-4 level was estimated using L-spine MRI: about 65 mm and 18 mm, respectively. We marked a 70 mm scale on the spinal needle, pierced the skin with it, and advanced it further until CSF came out through the needle. Since we thought that the needle was in the cyst, it was advanced more deeply. When no more CSF dropped out from the needle, we assumed that its tip was in the soft tissue between the pseudomeningocele and the dura. Then, we advanced the needle a little bit further until CSF again flowed freely and we reckoned that the needle had reached the true subarachnoid space. The needle’s remaining part was about 20 mm, so its insertion part was about 70 mm. The length of the needle was 89 mm. Hyperbaric 0.5% bupivacaine 10 mg with fentanyl 10 μg was slowly injected, while making sure that the patient felt no sensory abnormalities on her leg. This time, the anesthesia was successful. The level of sensory block reached to T7. Even though spinal anesthesia was performed twice, the level of sensory block maintained T7-T10 during surgery. When discharged from the post-anesthetic care unit, the level of sensory block was T12. The patient fully recovered from the sensory and motor block during the evening of the surgery. After discharge from the hospital, she decided to return to our medical institution in case of further spine surgeries.
Spinal anesthesia is a definitive and trustworthy anesthetic method, but it can sometimes unexpectedly fail. Most anesthesiologists believe that the incidence of spinal anesthesia failure is extremely low, but this varies widely among studies and has been reported to be as high as 17% [5]. However, with careful attention, anesthesiologists can avoid expectable failure and drop, lowering the incidence of spinal anesthesia failure to about 4% [6]. There are several causes of the failure: unsuccessful lumbar punctures, solution injection errors, inadequate intrathecal spreads, ineffective drug actions, and inadequate management [7]. Solution injection errors include wrong dose selections, loss of local anesthetics caused by disjoints between needles and syringes, as well as misplaced injections. Inadequate intrathecal spread is due to anatomical abnormalities such as kyphosis, scoliosis, and solution density while ineffective drug actions are caused by identification errors, chemical incompatibilities, inactive local anesthetic solutions, and local anesthetic resistance [7].
The reasons for failed lumbar punctures are poor positioning of the patients or incorrect needle insertions in addition to being induced by pseudo-successful lumbar punctures where clear fluid appears at the needle hub, but this is not CSF. It is either the local anesthetic infiltrated in the needle insertion area or fluid in an arachnoid cyst. Although pseudomeningocele is different from arachnoid cysts, it can also lead to pseudo-successful lumbar punctures.
Pseudomeningocele constitutes extradural CSF collection resulting from a dural tear and is similar to arachnoid cysts but differs in that the former consists of scar tissues and not of arachniod lines or dural covering [4]. The causes of pseudomeningoceles are congenital, traumatic, or iatrogenic. In this case, the cause of pseudomeningoceles seems to be the iatrogenic followed by spine surgery. Iatrogenic pseudomeningocele is mostly generated after durotomy during spine surgery [1]. The true incidence of iatrogenic pseudomeningoceles after spine surgery is unknown because most patients have no symptoms, but it is assumed to range from 0.1% to 2% among spine surgery patients according to previous studies [2,3]. A higher prevalence in the lumbar spine is not only caused by an elevated incidence of lumbar laminectomies but also by increased CSF pressure in lumbar areas [8]. Although there are generally no symptoms, pseudomeningocele can be present with a variety of signs and symptoms, such as low back pain, radicular pain, posture related headache, cervical or occipital pain, nausea, vomiting, myelopathy, and palpable mass. MRI is the most reliable tool for its diagnosis [9]. Different assessment modalities for pseudomeningocele should be conducted to distinguish it from delayed recurrences of radiculopathy or myelopathy after spinal procedures [10].
In our case, the patient had no symptoms, such as back pain or radicular pain. Therefore, we could not predict the presence of complications related to previous spine surgeries. Pseudomeningoceles was not suspected until the patient had reported it. She had already known that there was a pseudomeningocele in a previous operation site because the last surgeon had told her about it after the spine surgery. After admission to our hospital, the patient informed the orthopedic surgeon about the presence of pseudomeningocele but he failed to record and to tell the anesthesiologist about it. The patient also did not mention pseudomeningocele further when an anesthesiologist met her on a preoperative visit.
When we first tried spinal anesthesia, we found a drainage and aspiration of clear liquid and thought that the needle was in the subarachnoid space. However, the needle was actually in the pseudomeningocele and we performed a pseudo-successful lumbar puncture. This situation was repeated in the second attempt after which we were informed about the presence of pseudomeningocele and had to decide between repeated spinal anesthesia and general anesthesia. After MRI examination, no communication between the subarachnoid space and pseudomeningocele was evident and even if there had been one, the fistula was assumed to be very small, so the pseudomeningocele was formed by a ball valve mechanism that allows a one-way flow of CSF from the subarachnoid space to the pseudomeningocele [11,12]. Therefore, even if an additional local anesthetic had been administered, the drug would not have easily entered the subarachnoid space. Because the pseudomeningocele is composed of scar tissues, it is thought that the blood supply is poor and therefore, it systemic toxicity would not readily happen, even if an overdose of a local anesthetic had been lodged in the cyst. Hence, spinal anesthesia was repeated. We inserted the needle by considering the size of the cyst and eventually succeeded with spinal anesthesia. Because of the reasons above, there were no toxicities related to local anesthetics.
Pseudomeningocele is very rare, but it can become a reason for spinal anesthesia failure. In this case report, it can be witnessed that pseudomeningocele should be suspected if spinal anesthesia is not successful in patients who undergo spinal surgery. In addition, careful history taking, reviews of medical records before surgery and anesthesia, as well as close communication between surgeons and anesthesiologists are crucial for successful spinal anesthesia.
REFERENCES
1. Hawk MW, Kim KD. Review of spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2000; 9:e5. DOI: 10.3171/foc.2000.9.1.5. PMID: 16859266.
2. Schumacher HW, Wassmann H, Podlinski C. Pseudomeningocele of the lumbar spine. Surg Neurol. 1988; 29:77–8. DOI: 10.1016/0090-3019(88)90127-9.
3. Teplick JG, Peyster RG, Teplick SK, Goodman LR, Haskin ME. CT Identification of postlaminectomy pseudomeningocele. AJR Am J Roentgenol. 1983; 140:1203–6. DOI: 10.2214/ajr.140.6.1203. PMID: 6602493.
4. Macki M, Lo SF, Bydon M, Kaloostian P, Bydon A. Post-surgical thoracic pseudomeningocele causing spinal cord compression. J Clin Neurosci. 2014; 21:367–72. DOI: 10.1016/j.jocn.2013.05.004. PMID: 24210805.
5. Levy JH, Islas JA, Ghia JN, Turnbull C. A retrospective study of the incidence and causes of failed spinal anesthetics in a university hospital. Anesth Analg. 1985; 64:705–10. DOI: 10.1213/00000539-198507000-00010. PMID: 4014732.
6. Munhall RJ, Sukhani R, Winnie AP. Incidence and etiology of failed spinal anesthetics in a university hospital: a prospective study. Anesth Analg. 1988; 67:843–8. DOI: 10.1213/00000539-198809000-00008. PMID: 3414995.
7. Fettes PD, Jansson JR, Wildsmith JA. Failed spinal anaesthesia: mechanisms, management, and prevention. Br J Anaesth. 2009; 102:739–48. DOI: 10.1093/bja/aep096. PMID: 19420004.
8. Chung JY, Lee BJ, Kim YI, Kim YM, Yi JW, Kang JM, et al. Pseudomeningocele after lumbar discectomy treated with fibrin glue and epidural blood patch: a case report. Korean J Anesthesiol. 2009; 57:789–92. DOI: 10.4097/kjae.2009.57.6.789.
9. Netra R, Min L, Shao Hui M, Wang JC, Bin Y, Ming Z. Spinal extradural meningeal cysts: an MRI evaluation of a case series and literature review. J Spinal Disord Tech. 2011; 24:132–6. DOI: 10.1097/BSD.0b013e3181e47b47. PMID: 21430498.
10. Kamali R, Naderi Beni Z, Naderi Beni A, Forouzandeh M. Postlaminectomy lumbar pseudomeningocele with nerve root entrapment: a case report with review of literature. Eur J Orthop Surg Traumatol. 2012; 22(Suppl 1):57–61. DOI: 10.1007/s00590-011-0934-3. PMID: 26662749.
11. Cobb C 3rd, Ehni G. Herniation of the spinal cord into an iatrogenic meningocele. Case report. J Neurosurg. 1973; 39:533–6. DOI: 10.3171/jns.1973.39.4.0533. PMID: 4730343.
12. Bhatoe HS. Postoperative lumbar pseudomeningocele. J Indian Med Assoc. 1995; 93:280. 282.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download