Abstract
Iatrogenic postintubation tracheal injury is a rare but potentially fatal complication associated with anesthesia. However, as signs of tracheal injury including subcutaneous emphysema, pneumomediastinum, pneumothorax, and respiratory distress may also be related to surgical technique, diagnosis may be confused and treatment of tracheal injury can be delayed. We report a case of postintubation tracheal laceration, whose diagnosis was delayed because of symptoms were confused with subcutaneous emphysema after septorhinoplasty including osteotomy. As symptoms deteriorated in spite of conventional management, patient underwent evaluation to determine other causes and eventually postintubation tracheal injury was detected. Therefore, even if there is no problem during tracheal intubation, it is necessary to consider postintubation tracheal injury in patients with subcutaneous emphysema that worsens despite appropriate treatment after septorhinoplasty including osteotomy.
Postintubation tracheal laceration (PITL, or iatrogenic tracheal laceration) is a rare but potentially life-threatening complication associated with anesthesia. Signs of tracheal laceration include subcutaneous or mediastinal emphysema, and pneumothorax [1]. However, if lesions of laceration only involve mucosal or submucosal membrane, clinical signs can be minor and it may be hard to detect the injuries [2]. Besides, diagnosis of laceration may be delayed, because initial signs of tracheal laceration are like facial edema and subcutaneous emphysema that may occur from surgical procedures. After written informed consent was obtained from the patient, here we report a case of delayed diagnosis of PITL in a patient who underwent septorhinoplasty with osteotomy.
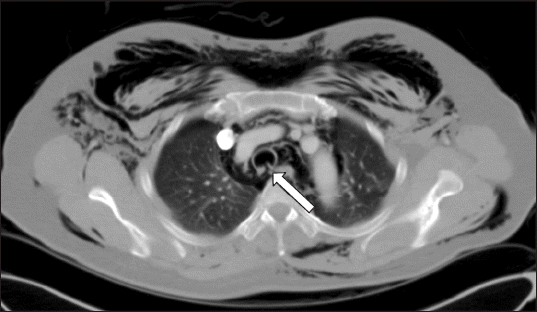
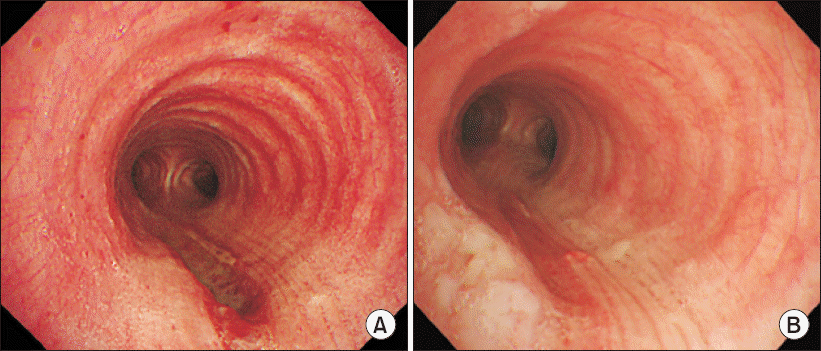
A 52-year-old female presented for right side nasal obstruction that started 2 years ago after nasal injury. She was diagnosed with septal deviation to right and C shaped external nose deviation. Her height was 160 cm and weight was 68 kg (body mass index was 26.5 kg/m2). She had taken medications for hypertension and preoperative evaluation did not show any specific abnormality. She underwent elective septorhinoplasty with osteotomy under general anesthesia. Anesthesia was induced with 300 mg of thiopental, 40 mg of rocuronium and a continuous infusion of remifentanil. Endotracheal intubation was performed with a 7.0-mm wired endotracheal tube. Her Mallampati score and laryngoscope grade were assessed as 1 and intubation was completed at first try without stylet use. Endotracheal tube cuff was manually inflated and pressure was not measured with a pressure gauge. During the operation, anesthesia was maintained with oxygen (FiO2 0.5) with air, desflurane and a continuous infusion of remifentanil. Hemitransfixion incision was made and subperichondrial plane was dissected. As there was still caudal deviation after removing the deviated portion of perpendicular ethmoid plate, septal cartilage was removed except for L strut and septal batten graft was performed using perpendicular ethmoid plate. To correct the external nose deviation, medial osteotomies were performed at nasal bone and lateral osteotomies were carried out at maxilla bone. Crushed septal cartilage was inserted at depressed dorsum for camouflage. Non-absorbable nasal packs were inserted and dorsal splint was applied. There were no adverse events during surgery and tracheal extubation was conducted in the operating room. Observing in postanesthetic care unit, immediate postoperative complication was not noted. On postoperative day 1, periorbital bruise and facial swelling were observed, which can be often found in the patients who have undergone osteotomies. Although tactile crepitus on perinasal and maxillary area was unusually noted, she was closely observated and conservative management with antibiotics (cefmetazole 2 g, levofloxacin 750 mg), as she had no respiratory symptom and showed stable physical condition. On the next day, diffuse subcutaneous emphysema remained on her face and unusually expanded to her neck and thorax. Even after nasal packs were removed, subcutaneous emphysema spread out to the anterior thorax and swelling was aggravated at her face, neck, and thorax. She complained of severe pain (visual analogue scale 5) on the subcutaneous emphysema lesion. Chest and neck radiographic images showed diffuse subcutaneous emphysema in neck and the chest wall, as well as pneumomediastinum. Computed tomography scan of the chest revealed extensive subcutaneous emphysema in bilateral neck and the chest wall, pneumomediastinum and right pneumothorax, and wall defect of the trachea at T4 level (Fig. 1). A blow-hole incision and negative-pressure wound therapy (CuraVAC®; CGBio Inc., Korea) were conducted by thoracic surgeon at the left anterior chest below the clavicle to prevent expansion of the subcutaneous emphysema and to reduce severe pain on lesions. On postoperative day 3, bronchoscopy was performed and 4 cm linear laceration was revealed at the posterior tracheal wall from 5 cm below the vocal cord to 2 cm above the carina (Fig. 2A). The lesion was accompanied by mucosal defect and muscular layer was exposed. As the patient had stable vital signs and adequate respiratory status, she was closely observed with conservative management and additional management was not conducted. On the ninth day after surgery, subcutaneous emphysema was resolved and CuraVAC was removed. Complete blood count and inflammatory markers revealed normal levels. Follow-up bronchoscopy on postoperative day 13 demonstrated healed state of the lesion (Fig. 2B). The patient was discharged on postoperative day 14 without further complication. Endoscopic laryngeal examination revealed no evidence of late complication at a 3-month follow-up visit.
PITL associated with orotracheal intubation is rare and its incidence is approximately 0.005% [1]. Risk factors include old age, female, emergent intubation, usage of stylet during intubation, double-lumen tube insertion, prior pathological conditions of the trachea, and corticosteroid therapy [2]. Direct injury may occur after multiple and wild attempts of orotracheal intubation in emergency situations, especially when a stylet is used. Possible causes of laceration are use of an inadequate tube size, cuff overinflation, and movements of the inflated tube [3].
Signs of tracheal injuries include soft tissue or mediastinal emphysema, pneumothorax, hemoptysis, dyspnea, and respiratory failure. However, signs of tracheal injuries could be confused with surgical complications. As postoperative swelling or subcutaneous emphysema mainly occurs through the incision site during surgical procedure. Such complications in the cervico-facial area are often associated with maxillofacial trauma, head and neck surgery, dental extraction, as well as postoperative habitual performances of valsalva maneuver or coughing [4]. Although surgical procedures in septorhinoplasty may cause subcutaneous emphysema, it is extremely rare that only few cases have been reported in the literature [5,6]. During the procedure of osteotomy, air could enter subcutaneous tissue through the incision site and produce a 1-way valve effect with the same mechanism as in maxillofacial trauma. In most patients, subcutaneous emphysema caused by septorhinoplasty is usually resolved after nasal pack removal with bed rest and self-limited. In this patient, however, lesion of subcutaneous emphysema spread throughout the neck, arms, and chest, even after nasal pack removal. Therefore, further evaluation, such as computed tomography and bronchoscopy, were considered to find out causes other than surgical procedure.
Generally, most PITLs after general anesthesia occur in the pars membranacea of the cervicothoracic trachea and are suspected with chest radiography and/or computed tomography can be confirmed by bronchoscopy within 6 hours after tracheobronchial injury [7]. In our case, PITL was diagnosed on the third day after operation. Since subcutaneous emphysema ranged from face and neck, it seemed to be a complication of the osteotomy procedure in septorhinoplasty. The patient received conservative treatment, because she had stable vital signs and adequate respiratory status. Further evaluation was conducted after subcutaneous emphysema spreading to the chest. Diagnosis is confirmed by bronchoscopy, which revealing the site and extent of laceration. Cardillo et al. [2] suggested morphological classification of PITL. This classification system categorizes PITL to Level I, II, IIIA, and IIIB, according to the depth of tracheal wall involvement. Level I is a mucosal or submucosal laceration without mediastinal emphysema or esophageal injury. Level II indicates laceration up to the muscular wall with subcutaneous or mediastinal emphysema without esophageal injury or mediastinitis. Level IIIA injury is complete laceration of the tracheal wall with esophageal or mediastinal soft tissue hernia without esophageal injury or mediastinitis. Level IIIB implies any laceration of the tracheal wall with esophageal injury or mediastinitis. PITL in our patient was evaluated as level II since mediastinal emphysema was observed but not esophageal involvement or mediastinitis.
Treatment of PITL depends on co-existing complications. Levels I and II lacerations with less than 5 cm in length are recommended for non-operative management. Surgical repair is indicated when full-thickness tracheal laceration with respiratory instability is detected or there is evidence of mediastinitis [8,9]. Conservative management includes bed rest, pain control, ventilatory support, and close observation. In patients with mild dyspnea, respiratory support using a non-invasive positive pressure ventilation can be helpful. When mechanical ventilation is applied, the tip of the endotracheal tube should be placed distal to the laceration. If the site of laceration is too close to the carina, separate endobronchial intubation may be necessary [10]. Intravenous broad-spectrum antibiotic therapy is also suggested for preventing mediastinitis. In most cases, spontaneous healing is achieved at 6 weeks after laceration [2]. Tracheal stricture and granulation tissue formation are significant long-term complications in PITL patients. Patients managed non-operatively do not need laser treatment, airway resection, endobronchial stent, or other management for strictures [11]. However, in patients who underwent surgical tracheal repair, strictures caused by granulation tissue formation have been reported. Therefore, conservative treatment is prior in clinically stable patients and this management strategy may be helpful for reducing long-term complications and requirement of granulation tissue debridement.
Negative-pressure wound therapy (NPWT) may be helpful for severe subcutaneous emphysema lesion. To conduct therapy, ‘Blow-hole’ incision is attempted to create an opening in the epidermal barrier and negative-pressure is applied at the area of lesion to prevent extension of subcutaneous emphysema. NPWT is to generate a pressure gradient, which removes air and fluid, reduces bacterial load, and improves healing by reducing tissue edema and stimulating fibroblast recruitment [12]. Complications associated with this therapy are rare, but a few cases of wound infection and bleeding have been reported [13]. In our patient, subcutaneous emphysema improved within 24 hours after NPWT had been applied. Additional management was not needed even after a 4 cm level II tracheal laceration was confirmed by bronchoscopic examination, as emphysema were reduced without signs of infection such as mediastinitis.
In conclusion, subcutaneous emphysema is often observed at postoperative period of septorhinoplasty including osteotomy and could be well-managed conservatively. However, if symptoms worsen even with appropriate treatment, other causes of subcutaneous emphysema should be considered. PITL may be a cause of gradually expanding emphysema that could progress to severe pneumomediastinum or pneumothorax leading to dyspnea. To select adequate endotracheal tube size, to inflate the cuff with appropriate pressure, and to avoid excessive force indwelling endotracheal tube can have significant role in reducing incidence of PITL. As seen in our case, however, PITL may occur even though intubation is uneventfully conducted. Anesthesiologists should be aware of the possibility of PITL when emphysema expands and should prevent delay in diagnosis and management.
ACKNOWLEDGMENTS
This work was supported by clinical research grant from Pusan National University Yangsan Hospital in 2017.
REFERENCES
1. Massard G, Rougé C, Dabbagh A, Kessler R, Hentz JG, Roeslin N, et al. Tracheobronchial lacerations after intubation and tracheostomy. Ann Thorac Surg. 1996; 61:1483–7. DOI: 10.1016/0003-4975(96)00083-5.
2. Cardillo G, Carbone L, Carleo F, Batzella S, Jacono RD, Lucantoni G, et al. Tracheal lacerations after endotracheal intubation: a proposed morphological classification to guide non-surgical treatment. Eur J Cardiothorac Surg. 2010; 37:581–7. DOI: 10.1016/j.ejcts.2009.07.034. PMID: 19748275.
3. Hussein E, Pathak V, Shepherd RW, Shojaee S. Bronchoscopic management of iatrogenic tracheal laceration using polyurethane-covered nitinol tracheal stents. J Trauma Acute Care Surg. 2016; 81:979–83. DOI: 10.1097/TA.0000000000001233. PMID: 27602904.
4. Durukan P, Salt O, Ozkan S, Durukan B, Kavalci C. Cervicofacial emphysema and pneumomediastinum after a high-speed air drill endodontic treatment procedure. Am J Emerg Med. 2012; 30:2095. DOI: 10.1016/j.ajem.2012.01.006. PMID: 22306391.
5. Kim ES, Kang JW, Kim CH, Hong JM. Cervical subcutaneous emphysema and pneumomediastinum after septorhinoplasty. J Craniofac Surg. 2014; 25:533–4. DOI: 10.1097/SCS.0000000000000693. PMID: 24577299.
6. Celebioğlu S, Keser A, Ortak T. An unusual complication of rhinoplasty: subcutaneous emphysema. Br J Plast Surg. 1998; 51:266–7. DOI: 10.1016/S0007-1226(98)80027-1.
7. Carbognani P, Bobbio A, Cattelani L, Internullo E, Caporale D, Rusca M. Management of postintubation membranous tracheal rupture. Ann Thorac Surg. 2004; 77:406–9. DOI: 10.1016/S0003-4975(03)01344-4.
8. Jougon J, Ballester M, Choukroun E, Dubrez J, Reboul G, Velly JF. Conservative treatment for postintubation tracheobronchial rupture. Ann Thorac Surg. 2000; 69:216–20. DOI: 10.1016/S0003-4975(99)01129-7.
9. Denlinger CE, Veeramachaneni N, Krupnick AS, Patterson GA, Kreisel D. Nonoperative management of large tracheal injuries. J Thorac Cardiovasc Surg. 2008; 136:782–3. DOI: 10.1016/j.jtcvs.2007.12.040. PMID: 18805287.
10. Conti M, Pougeoise M, Wurtz A, Porte H, Fourrier F, Ramon P, et al. Management of postintubation tracheobronchial ruptures. Chest. 2006; 130:412–8. DOI: 10.1378/chest.130.2.412. PMID: 16899839.
11. Gómez-Caro A, Ausín P, Moradiellos FJ, Díaz-Hellín V, Larrú E, Pérez JA, et al. Role of conservative medical management of tracheobronchial injuries. J Trauma. 2006; 61:1426–34. DOI: 10.1097/01.ta.0000196801.52594.b5. PMID: 17159686.
12. Towe C, Solomon B, Donington JS, Pass HI. Treatment of recalcitrant subcutaneous emphysema using negative pressure wound therapy dressings. BMJ Case Rep 2014. 2014; bcr2014205577.
13. Kim PJ, Attinger CE, Olawoye O, Crist BD, Gabriel A, Galiano RD, et al. Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds. 2015; 27:S2–19.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download