INTRODUCTION
MATERIALS AND METHODS
Animal preparation
Drugs
Nociceptive test
Postoperative pain model
Inflammatory pain model
Behavioral pain testing
Experimental protocol
Effects of curcumin on postoperative and inflammatory pain
GABA and opioid receptor expression upon induction of postoperative and inflammatory pain
Roles played by GABA or opioid receptor subtypes in the effects of curcumin on postoperative and inflammatory pain
General behavior
Statistical analysis
RESULTS
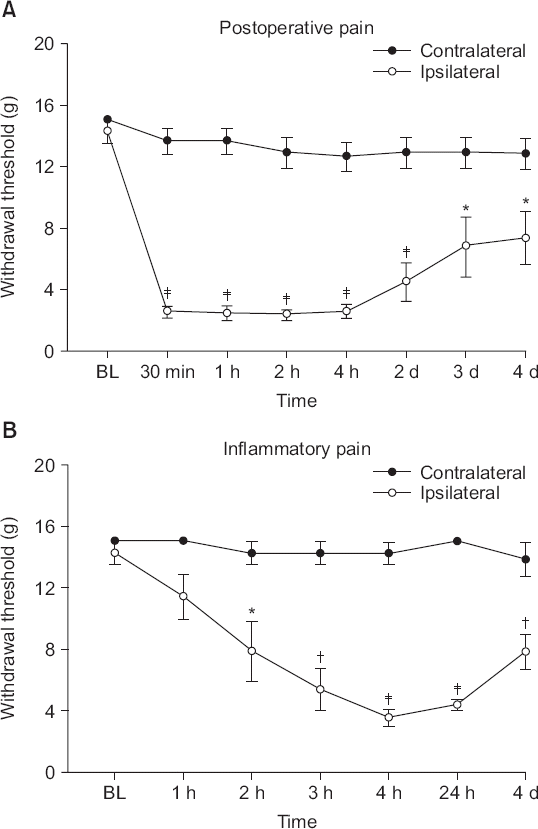 | Fig. 1Time course of hindpaw withdrawal responses to von Frey filament pressure after (A) incisional surgery or (B) carrageenan injection. Data are presented as withdrawal thresholds. Each line represents the mean ± SEM of data from five or six rats. BL: baseline withdrawal threshold measured prior to paw incision or carrageenan injection. Significant differences between data from the ipsilateral and contralateral sites are indicated, *P < 0.05, †P < 0.01, ‡P < 0.001. |
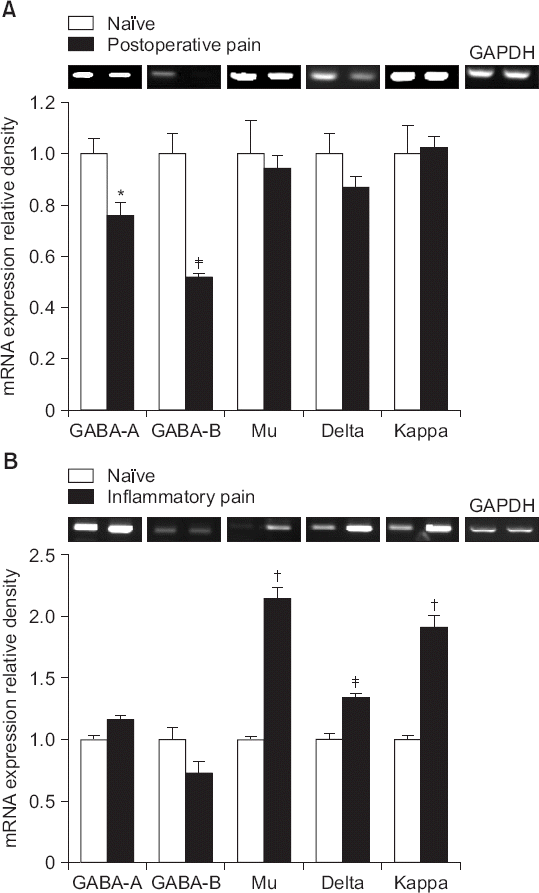 | Fig. 2Expression levels of mRNAs encoding γ-aminobutyric acid (GABA) and opioid receptors in the left dorsal horn of the spinal cord in naïve, incised, and carrageenan-injected rats. Data are presented as relative values of GABA and opioid receptors to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each bar represents the mean ± SEM of data from four rats. Reverse transcriptase polymerase chain reaction detected mRNA bands corresponding to the GABA and opioid receptors in naïve animals. (A) Quantitative analysis indicated that incisional surgery reduced the levels of expression of mRNAs encoding GABA receptors, but not of those encoding opioid receptors. (B) Carrageenan injection increased expression of opioid receptor mRNAs, but not those of GABA receptor mRNAs. *P < 0.05, †P < 0.01, ‡P < 0.001 compared to naïve rats. |
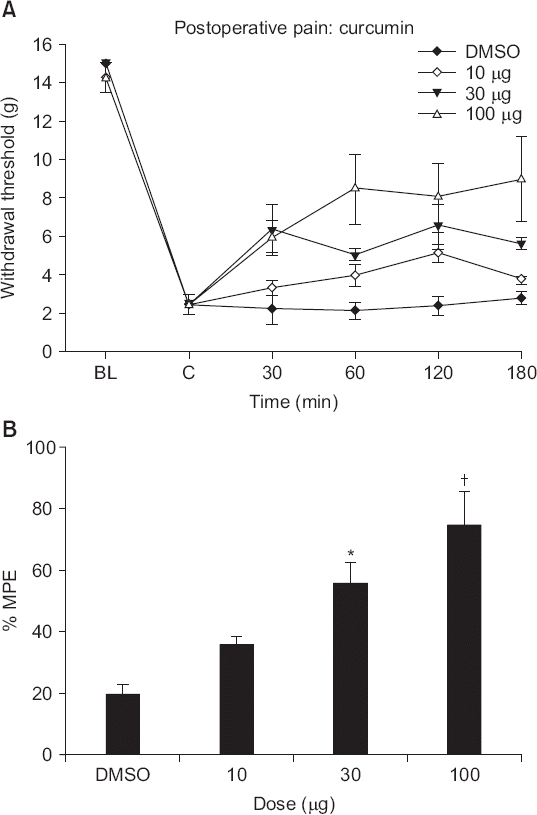 | Fig. 3(A) The time effect and (B) dose-response of intrathecal curcumin on the hindpaw withdrawal response to von Frey filament pressure applied after incisional surgery. Data are presented as withdrawal thresholds or percentages of maximal possible effects (%MPEs). Each line represents the mean ± SEM of data from five-to-seven rats. Control data were obtained 2 h after incision. Curcumin was administered immediately after measurement of control post-incisional thresholds. BL: baseline withdrawal threshold measured before paw incision. Curcumin produced dose-dependent increases in withdrawal thresholds of injured paws after incision. *P < 0.05, †P < 0.01 compared to vehicle (dimethylsulfoxide, DMSO) alone. |
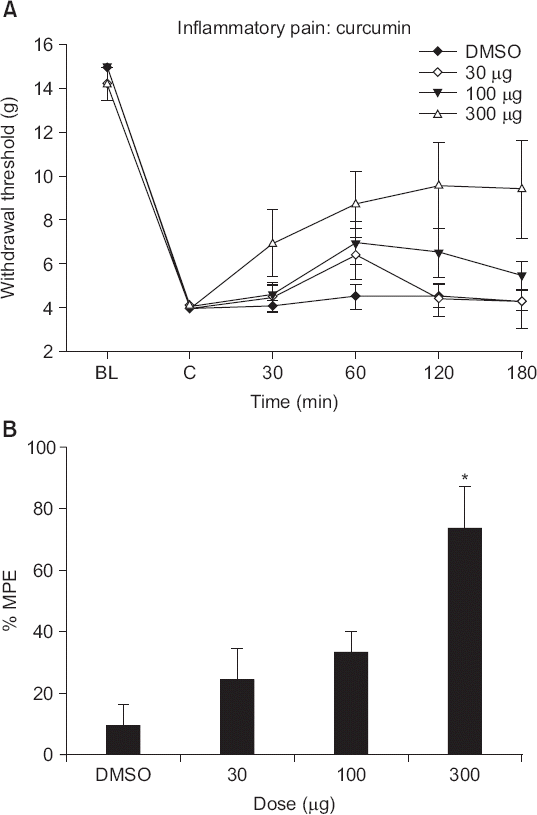 | Fig. 4(A) The time effect of and (B) dose-response to intrathecal curcumin, assessed by measuring hindpaw withdrawal responses to von Frey filament pressure applied after carrageenan injection. Data are presented as withdrawal thresholds or percentages of maximal possible effects (%MPEs). Each line represents the mean ± SEM of data from five-to-seven rats. Control data were obtained 4 h after carrageenan injection. Curcumin was administered immediately after measurement of control post-injectional thresholds. BL: baseline withdrawal threshold measured before carrageenan injection. Curcumin caused a dose-dependent increase in the withdrawal thresholds of injected paws after carrageenan injection. *P < 0.05 compared to vehicle (dimethylsulfoxide, DMSO) alone. |
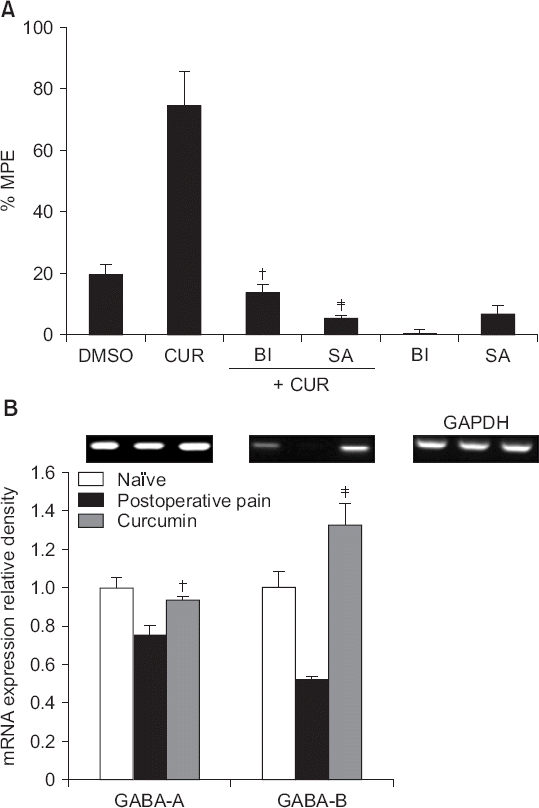 | Fig. 5(A) The effects of intrathecal bicuculline (a γ-aminobutyric acid [GABA]-A receptor antagonist, 0.3 μg), and saclofen (a GABA-B receptor antagonist, 100 μg), on the antinociceptive effects of intrathecal curcumin (100 μg) in rats experiencing postoperative pain; and (B) the effects of intrathecal curcumin (100 μg) on the expression levels of mRNAs encoding GABA receptors in the left dorsal horn of the spinal cord in incised rats. Data are presented as percentages of maximal possible effects (%MPEs) or the relative values of GABA receptor levels to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each bar represents the mean ± SEM of data from four-to-six rats. DMSO: dimethylsulfoxide, CUR: curcumin, BI: Bicuculline, SA: saclofen. (A) Both bicuculline and saclofen reversed the effects of curcumin, †P < 0.01, ‡P < 0.001 compared with curcumin. (B) Intrathecal curcumin increased the expression levels of mRNAs encoding GABA receptors, †P < 0.01, ‡P < 0.001 compared with the incised group. |
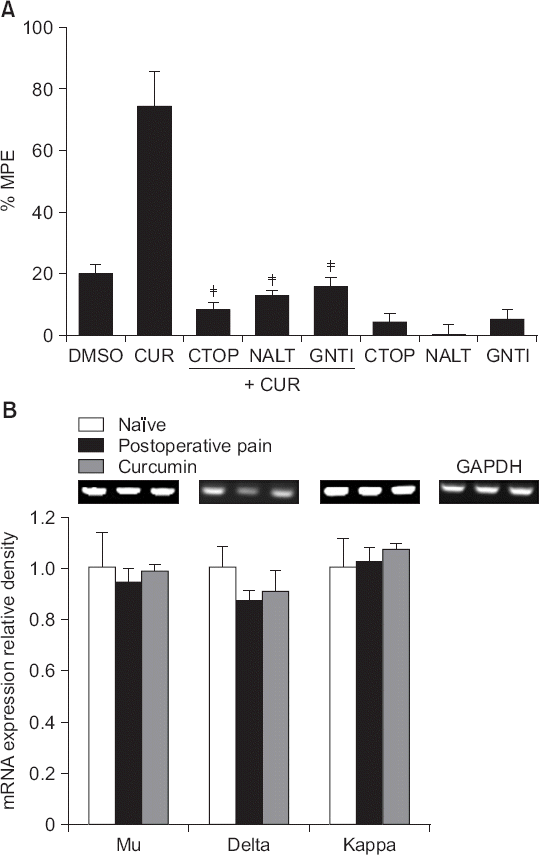 | Fig. 6(A) The effects of intrathecal CTOP (a μ opioid receptor antagonist, 15 μg), naltrindole (a δ opioid receptor antagonist, 10 g), and GNTI (a κ opioid receptor antagonist, 50 μg) on the antinociceptive effects of intrathecal curcumin (100 μg) on postoperative pain, and (B) the effects of intrathecal curcumin (100 μg) on the expression levels of mRNAs encoding opioid receptors in the left dorsal horn of the spinal cord in incised rats. Data are presented as percentages of maximal possible effects (%MPEs) or the relative expression levels of opioid receptors compared to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each bar represents the mean ± SEM of data from four-to-six rats. DMSO: dimethylsulfoxide, CUR: curcumin, NALT: naltrindole. (A) CTOP, naltrindole and GNTI reversed the effects of curcumin, ‡P < 0.001 compared with curcumin. (B) Intrathecal curcumin only marginally affected the expression levels of mRNAs encoding opioid receptors in the incised group. |
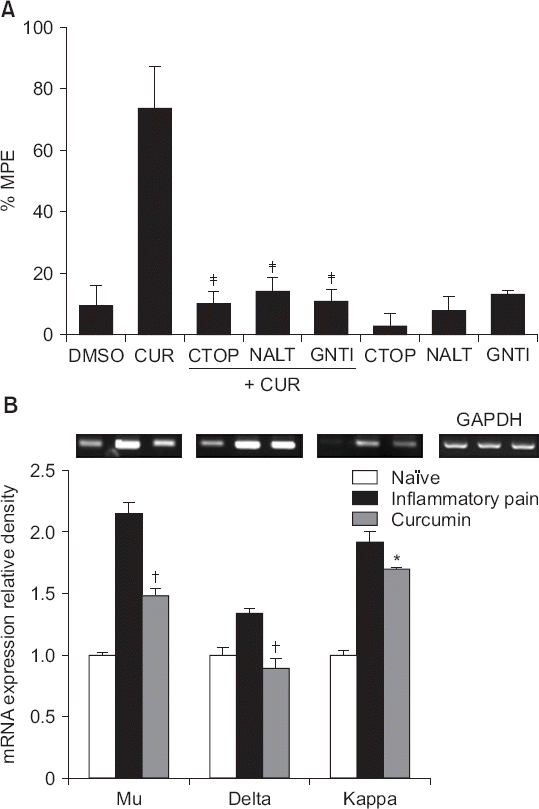 | Fig. 7(A) The effects of intrathecal CTOP (a μ opioid receptor antagonist, 15 μg), naltrindole (a δ opioid receptor antagonist, 10 μg) and GNTI (a κ opioid receptor antagonist, 50 μg) on the antinociceptive effects of intrathecal curcumin (300 μg) on inflammatory pain, and (B) the effects of intrathecal curcumin (300 μg) on the expression levels of mRNAs encoding opioid receptors in the left dorsal horn of the spinal cord in carrageenan-injected rats. Data are presented as percentages of maximal possible effects (%MPEs) or the relative expression levels of opioid receptors compared to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each bar represents the mean ± SEM of data from four-to-six rats. DMSO: dimethylsulfoxide, CUR: curcumin, NALT: naltrindole. (A) All of CTOP, naltrindole, and GNTI reversed the effects of curcumin. ‡P < 0.001 compared with curcumin. (B) Intrathecal curcumin decreased the expression levels of mRNAs encoding all three opioid receptors, *P < 0.05, †P < 0.01, compared to the inflammatory group. |
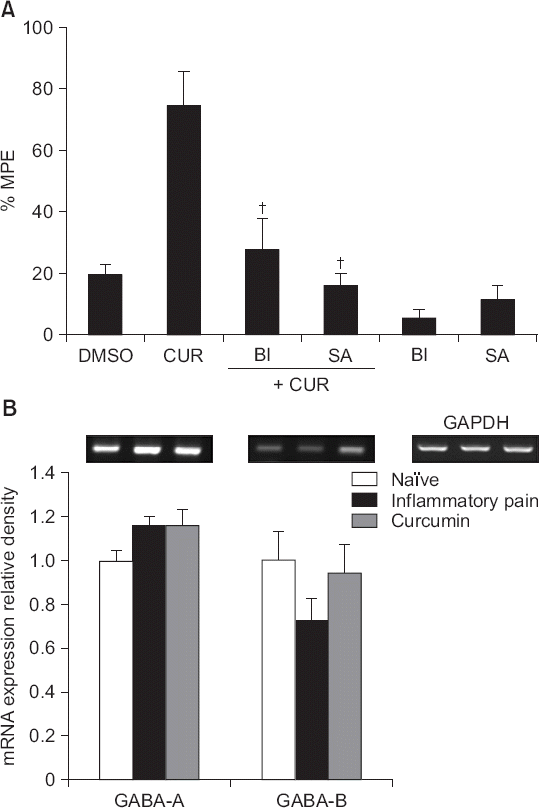 | Fig. 8(A) The effects of intrathecal bicuculline (a γ-aminobutyric acid [GABA]-A receptor antagonist, 0.3 μg) and saclofen (a GABA-B receptor antagonist, 100 μg) on the antinociceptive effects of intrathecal curcumin (300 μg) on inflammatory pain, and (B) the effects of intrathecal curcumin (300 μg) on the expression levels of mRNAs encoding GABA receptors in the left dorsal horn of the spinal cord in carrageenan-injected rats. Data are presented as percentages of maximal possible effects (%MPEs) or the relative expression levels of GABA receptors to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each bar represents the mean ± SEM of data from four-to-six rats. DMSO: dimethylsulfoxide, CUR: curcumin, BI: Bicuculline, SA: saclofen. (A) Both bicuculline and saclofen reversed the effects of curcumin. †P < 0.01 compared with curcumin. (B) Intrathecal curcumin only marginally affected the expression levels of mRNAs encoding GABA receptors in the inflammatory group. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download