INTRODUCTION
Vena cava syndrome is a condition presenting with various symptoms caused by central venous obstruction, which can be divided into superior vena cava (SVC) syndrome and inferior vena cava (IVC) syndrome [1]. Although IVC syndrome is not as well-known as SVC syndrome, IVC obstruction can lead to superficial venous dilation as well as abdominal and lower limb edema [2]. It can have a diverse presentation, ranging from no symptoms to bilateral lower limb edema, hypotension, and supine hypotensive syndrome [2,3].
Laparoscopic surgery is widely used to operate on various abdominal organs. Because carbon dioxide gas is insufflated intra-abdominally and the patient’s position is changed in order to obtain the required field of view, the anesthesiologist needs to be vigilant regarding changes in the patient’s vital signs during surgery.
Although case reports of perioperative SVC syndrome are common, perioperative IVC syndrome has been rarely reported [4,5]. Here, we present a case of sustained hypotension due to compression of the IVC by intrathoracic herniation of peritoneal fat during laparoscopic anterior resection, along with a review of the literature.
CASE REPORT
A 65-year-old man (height, 161 cm; weight, 81 kg) was scheduled for a laparoscopic anterior resection after being diagnosed as having sigmoid colon cancer. The patient had no specific history except for hypertension. Preoperative chest radiography, pulmonary function tests, and electrocardiography showed normal findings, while echocardiography showed a left ventricular ejection fraction of 55%, grade 1 diastolic dysfunction, and left atrial enlargement. His hemoglobin level was 12.1 g/dl and his hematocrit level was reduced to 37.6%. His height was 161 cm, body weight was 81 kg, and abdominal circumference was 102 cm. His systolic blood pressure (SBP) was 105 to 135 mmHg, diastolic blood pressure (DBP) was 65 to 85 mmHg, and heart rate (HR) was 65 to 85 beats/min in the ward.
Immediately after arriving at the operating room, the patient’s blood pressure was 109/79 mmHg, HR was 89 beats/min, respiration rate (RR) was 15 breaths/min, and peripheral arterial oxygen saturation was 95%. Anesthesia was induced using 40 mg lidocaine, 100 mg propofol, and 50 mg rocuronium. Following 3 minutes of mask ventilation with sevoflurane and 100% oxygen, a 7.5-mm diameter endotracheal tube was placed in the trachea. Anesthesia was maintained by 1.1 L/min oxygen, 1.9 L/min medical air, 2 vol% sevoflurane, continuous infusion of remifentanil (1.0–5.0 ng/ml), and intermittent infusion of 10 to 20 mg rocuronium. An arterial line was inserted in the right radial artery for continuous blood pressure monitoring and blood sampling for arterial blood gas analysis. An additional intravenous route was placed in the left external jugular vein.
The ventilator was set at tidal volume of 600 ml, RR of 10 breaths/min, with no positive end-expiratory pressure, and the peak inspiratory pressure (PIP) was 23 cmH2O. After induction of anesthesia, the patient’s body was placed in the lithotomy-Trendelenburg position. During surgery, carbon dioxide was continuously insufflated into the peritoneal cavity to aid laparoscopy, with the intraperitoneal pressure maintained at 13 mmHg. Tidal volume and RR were controlled to maintain partial pressure of carbon dioxide (PaCO2) in the arterial blood at 35 to 45 mmHg. Arterial blood gas analysis immediately after induction of anesthesia showed the following results: fraction of inspired oxygen, 0.5; pH, 7.493; PaCO2, 34.8 mmHg; partial pressure of oxygen (PaO2), 159 mmHg; and base excess, 3 mEq/L. After approximately 10 minutes of intraperitoneal insufflation, PIP rose rapidly to 35 cmH2O. Auscultation revealed wheezing in the right upper lung field; thus, 10 sprays of albuterol were applied in an endotracheal tube, and 100 mg hydrocortisone was injected intravenously. Subsequently, normal lung sounds were recovered, but PIP remained at 35 cmH2O; thus, the ventilator settings were adjusted to tidal volume of 420 ml and RR of 16 breaths/min. Because no other causes could be determined by physical examination, the elevation in PIP was considered to be due to the patient being overweight; PIP was maintained at 32 to 35 cmH2O thereafter.
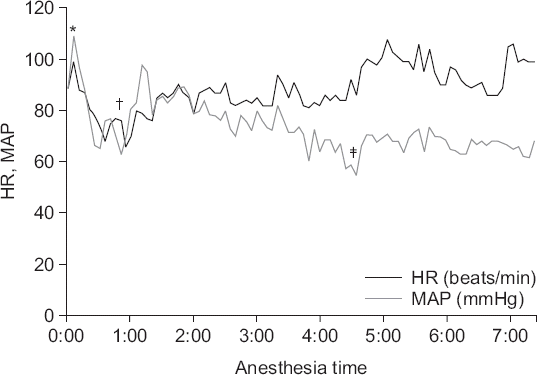
During the first 150 minutes of surgery, SBP was 95 to 125 mmHg, DBP was 55 to 85 mmHg, and HR was 60 to 85 beats/min. For the next hour, his blood pressure continued to decrease: SBP was 85 to 95 mmHg, DBP was 50 to 60 mmHg, and HR was 80 to 90 beats/min; thus, 100 μg phenylephrine was injected intermittently to maintain blood pressure. When the decrease in blood pressure became more severe, dopamine infusion was started at 5 to 8 μg/kg/min; SBP was 80 to 100 mmHg, DBP was 45 to 60 mmHg, and HR was 85 to 100 beats/min. Minimal SBP was 76 mmHg, minimal DBP was 44 mmHg, and HR was 86 beats/min at that time. After deflation of the abdomen and supine positioning, hypotension was sustained. The total operating time was approximately 6 hours, and after completion of surgery and cessation of inhalational anesthetic, 200 mg sugammadex was infused to reverse muscle relaxation. After waiting 30 minutes for the patient to recover from anesthesia, his mental state was still drowsy; thus, he was moved to the intensive care unit (ICU) while maintaining endotracheal intubation. Even after stopping anesthesia, the patient’s SBP was 85 to 95 mmHg, DBP was 50 to 55 mmHg, and HR was 90 to 100 beats/min; therefore, continuous dopamine infusion was maintained at 5 μg/kg/min. The perioperative changes in the vital signs are illustrated in Fig. 1. The total intraoperatively infused fluid volume was 4,300 ml (500 ml colloid, 3,800 ml crystalloid), the intraoperative urine output was 565 ml, and estimated blood loss was 500 ml.
Fig. 1
Intraoperative changes in vital signs. HR: heart rate, MAP: mean arterial blood pressure. *Intubation, †start of surgery (start of carbon dioxide pneumoperitoneum and lithotomy-Trendelenburg position), ‡start of dopamine continuous infusion.
At the time of ICU admission, the patient breathed using a T-piece with FiO2 of 0.4. Twenty minutes after ICU admission, the patient recovered with an alert mental state, and the endotracheal tube was removed after confirming recovery from muscle relaxation using a grip test. Following ICU admission, the patient’s SBP was 100 to 135 mmHg, DBP was 50 to 70 mmHg, and HR was 80 to 105 beats/min. The dopamine dose was gradually reduced, and administration was stopped after 1 day. The patient’s vital signs stabilized and he was transferred to a general ward.
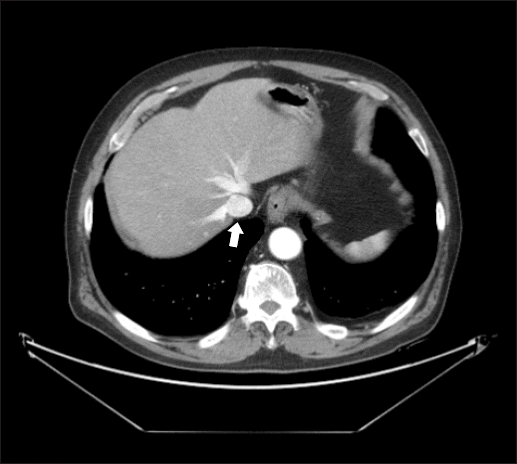
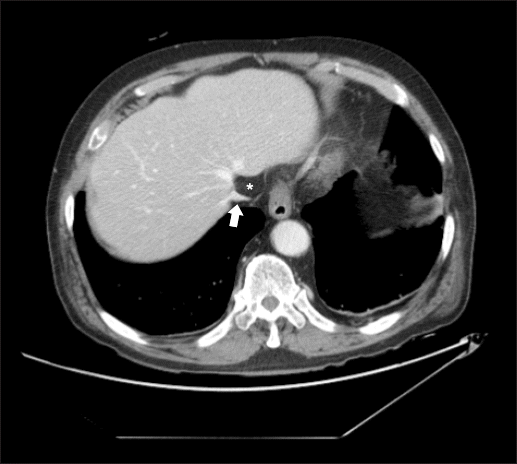
Preoperative abdomino-pelvic computed tomography (CT) showed a normal IVC and hepatic vein, not compressed by peritoneal fat (Fig. 2). The postoperative abdomino-pelvic CT, however, revealed compression of the IVC and hepatic vein due to intrathoracic herniation of peritoneal fat (Fig. 3). Although the patient had no signs of venous obstruction, such as lower limb edema, anticoagulation therapy was started on the recommendation of the Department of Thoracic Surgery. On postoperative day 16, the patient was discharged with no complications.
DISCUSSION
Vena cava syndrome is a rare group of symptoms produced by obstruction of the vena cava. Approximately 19,000 cases of SVC syndrome occur annually in the United States, whereas IVC syndrome is more rare; accurate statistical data are not available [6].
Causes of SVC syndrome include external factors, such as lymphoma, thymoma, lymph node expansion, and aortic aneurysm, as well as internal factors, such as thrombosis; however, approximately 95% of cases are caused by malignant disease [7,8]. SVC obstruction presents with symptoms of congestion and edema in the upper limbs, head, neck, and upper thoracic area, as well as dyspnea, dysphagia, and headache [8]. Diagnosis of SVC obstruction is made clinically and confirmed by CT [7,9]. Treatment consists of direct treatment of tumors using radiotherapy and/or chemotherapy, alleviation of edema using glucocorticoid therapy or loop diuretics, and sometimes, intravascular stenting or bypass surgery to resolve the obstruction [10].
Perioperative IVC syndrome has been rarely reported [4,5]. Most cases of IVC syndrome (> 90%) occur in the infrarenal segment. Internal causes of IVC obstruction include thrombosis, stenosis, primary tumor, and iatrogenic causes, while external causes include liver expansion due to hypertrophy, tumor or regenerating nodule, adenopathy, aortic aneurysm, pregnant uterus, and abdominal compartment syndrome [3]. In the present case, IVC syndrome was caused by the IVC becoming caught between the diaphragm and fat at the diaphragm level, which is at the border of the hepatic and renal segments; therefore, this can be considered as a very rare case. One possible cause for this rare situation is elevated intra-abdominal pressure due to the patient’s obesity, with a body mass index of 31.2 kg/m2 and abdominal circumference of 102 cm [11]. In addition, intra-abdominal pressure increased even further due to carbon dioxide insufflation to aid laparoscopy. Furthermore, the lithotomy-Trendelenburg position also is expected to have had an impact. The patient’s PIP was 23 cmH2O at the time of anesthesia induction, and increased to 33 to 35 cmH2O during surgery, even though tidal volume decreased after the start of surgery, indicating that intra-thoracic and intra-abdominal pressure were considerably elevated [12]. This elevated intra-abdominal pressure may have pushed the intraperitoneal fat towards the diaphragm, and likely played a pivotal role in causing the fat to move between the IVC and diaphragm.
The symptoms of IVC syndrome vary depending on the speed of the occlusion, condition of the collateral veins, and flow of the venous system. While cases of slow occlusion with intact collateral veins can be asymptomatic, acute occlusion can present with sudden bilateral lower limb edema and collateral vein enlargement. In some cases, hypotension develops due to a sudden decrease in venous return [2,3]. In the present case, a sudden intraoperative increase in intra-abdominal pressure led to the intraperitoneal fat being pushed towards the diaphragm and causing IVC obstruction, which, in turn, resulted in hypotension. IVC syndrome is diagnosed through a combination of physical examinations, such as observation of bilateral lower limb edema, changes in skin coloration, ulcers, and/or expansion of veins in the abdominal wall, as well as imaging studies, such as ultrasonography, CT, and/or magnetic resonance imaging. In this case, diagnosis was made on the basis of presence of hypotension and CT showing IVC compression. Although thrombus is a common cause of IVC syndrome, the mass compressing the IVC was located outside the IVC on CT and diagnosed as herniated peritoneal fat by a radiologist.
Treatment differs according to the etiology, but when acute thrombosis is the cause, treatment is similar to that of lower limb deep vein thrombosis. Anticoagulation therapy and compression stockings can be used, and there are almost no cases in which surgical thrombectomy is indicated; however, catheter thrombolysis is sometimes performed [3,13]. When the cause is compression by an external mass, the primary objective is to remove the mass, if possible. When it is a tumor, it can be removed surgically, or its size can be reduced through radiation and/or chemotherapy. Conservative treatment, including diuretics, compression stockings, anticoagulation, and antiplatelet therapy, also can be performed [3]. Treatment should be decided carefully according to the etiology and severity of symptoms because vena cava obstruction can resolve spontaneously [3,10]. In the present study, the patient did not report any symptoms, except for hypotension, which developed during surgery. Furthermore, the cause of IVC compression was herniated peritoneal fat. No specific therapy was implemented except anticoagulation therapy, which was recommended by the Department of Thoracic Surgery to prevent thrombosis from blood stasis in the narrowed IVC. The patient did not show any symptom related to IVC compression, and herniated fat was not observed on the 6-month follow-up CT. There are various causes of intraoperative hypotension. We assumed that the hypotension was caused by reduced contractility due to the anesthetics as well as reduced preload due to the elevated intra-abdominal pressure as a result of carbon dioxide insufflation. However, hypotension continued after the completion of surgery and the cause was not discovered; therefore, the patient was transferred to the ICU while maintaining continuous dopamine infusion. Subsequently, IVC compression was observed on CT, showing that the cause of hypotension was reduced venous return via the IVC.
A limitation of this case report is that the relationship between hypotension and IVC compression by fat has not been confirmed. In our case, hypotension was the only symptom observed, which resolved spontaneously. Therefore, we consider that hypotension may have been caused by the combined effect of carbon dioxide pneumoperitoneum, anesthesia, and IVC compression by peritoneal fat.
Although intraoperative hypotension occurred in the present case, the precise cause could not be found until IVC compression was observed on postoperative CT. It is often difficult to determine the exact cause of intraoperative hypotension, but when an obese patient undergoes laparoscopic surgery in the Trendelenburg position, as in the present case, IVC obstruction or compression should be considered.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download