INTRODUCTION
Adequate sedation and pain control are important for patients who require ventilatory support in a postoperative intensive care unit (ICU). Post-operative irritation and pain may increase coronary spasm and myocardial oxygen supply/consumption imbalance, by increasing blood catecholamines and may cause myocardial infarction, especially in patients with coronary artery disease [
1]. After the surgery, proper sedation and pain control stabilize the cardiovascular system to prevent myocardial infarction [
2], and improve patient prognosis by reducing the duration of mechanical ventilation and intensive care unit hospitalization [
3].
Dexmedetomidine is a highly selective alpha 2-adrenoreceptor agonist that can be used to reduce the use of analgesics, stabilize sedation and analgesic effects through continuous intravenous infusion [
4], and inhibit sympathetic activity and the secretion of catecholamines [
5]. It is widely used in the ICU to sedate the patients.
Previous studies on ICU patients showed no difference between the continuous infusion of dexmedetomidine and the infusion of midazolam in terms of reaching and sustaining sedation levels. Dexmedetomidine infusion resulted in a shorter duration of mechanical ventilation and decreased incidence of hypertension, tachycardia, and delirium [
6].
The protocol of this hospital used to maintain the sedation level in the intensive care unit after coronary artery bypass surgery was intravenous injection of midazolam. Midazolam was intravenously injected, with a bolus administered intermittently according to the patient’s sedative state, until the weaning from ventilatory support. The authors compared the intermittent administration of midazolam, which has been used previously, and the continuous infusion of dexmedetomidine, which had been increasingly used as a sedative in the intensive care unit. The authors performed a prospective randomized study for both groups where clinical outcomes such as the sedation status, pain control, and cardiac function were measured, after coronary artery bypass grafting. The authors evaluated the efficacy of dexmedetomidine for the sedation of patients after coronary artery bypass surgery in the intensive care unit.
Go to :

MATERIALS AND METHODS
This study was a prospective, randomized controlled, open-label, parallel group design clinical study, conducted after obtaining approval from the Institutional Review Board of Sanggye Paik Hospital and written consent from patients (IRB no. SPIRB-13-078). The researchers enrolled 60 patients, who underwent open-heart coronary artery bypass grafting, under general anesthesia from November 4, 2013 to May 9, 2016. Exclusion criteria were as follows: American Society of Anesthesiologists physical status V, emergency surgery, chronic sedative hypnotics, and combined valve surgery.
Glycopyrrolate was injected intramuscularly at 0.005–0.01 mg/kg for premedication, and the patients were anesthetized with total intravenous anesthesia using a target controlled infusion of propofol and remifentanil. Rocuronium was used as a muscle relaxant. In addition to standard monitoring, arterial and central venous pressure were monitored and a Swan-Ganz catheter was inserted to monitor pulmonary arterial pressure.
In this study, Richmond Agitation-Sedation Scale (RASS) was used. Based on awakened calm state (alert & calm), -1 means drowsy, -2 means light sedation, -3 means to respond to voice (moderate sedation). The authors set the target level of sedation (RASS range, −2 to −1) [
7].
In the dexmedetomidine group (DEX group), a continuous infusion of dexmedetomidine was started at the rate of 0.5–0.7 μg/kg/h without loading dose from the beginning of sternum closure and titrated to reach the target sedation level (RASS score, −2 to −1) in the intensive care unit. The patients in the midazolam group (MDZ group) received an intravenous bolus injection of midazolam 0.03–0.1 mg/kg, according to the patient’s age and hemodynamic status, at the time of sternum closure in the operation room. In both groups, an intravenous injection of 1 μg/kg of fentanyl was given two to three times with a 15-minutes interval, according to the hemodynamic status of the patient from the time of sternum closure until the end of anesthesia. In addition, a midazolam 0.03–0.1 mg/kg bolus was injected when RASS +2 was measured in the intensive care unit.
The primary end point was the percentage of time to reach the target range (RASS score, −2 to −1) and the secondary end points were the visual analog scale (VAS) for pain. All patients’ RASS, VAS for pain, cardiac index (CI), and hemodynamic changes (blood pressure, heart rate, and pulmonary arterial pressure) were recorded every two hours from the time of the study drug injection to the weaning from mechanical ventilation. The researchers compared the overall recovery status of patients with delirium (Confusion Assessment Method for the ICU [CAM-ICU]), duration of ICU admission, and duration of hospital stay. Ejection fractions (EF) were measured by transthoracic echocardiography (TTE) before and after surgery. Postoperative TTE was performed on fifth to eighth postoperative days.
In both groups, the pain was controlled through the intravenous patient controlled analgesia by mixing 1,500 μg of fentanyl, 80 mg of nefopam, and 60 ml of normal saline into a total volume of 100 ml. The basal infusion rate was 1 ml/h, the bolus dose was 0.5 ml, and the lockout interval was 10-minutes. The device used was an AutoMed 3200® (Ace Medical, Korea). When additional pain control was deemed necessary (VAS 7 or higher), 1 μg/kg of fentanyl was given intravenously in the intensive care unit. The pain level was measured on a conventional 10 cm visual analogue scale and confirmed by a visual and eye flicker. No other sedatives or narcotics were used during the study.
Electrocardiogram, blood test results, hemodynamic changes, physical examination, and adverse events were monitored. When hypotension and bradycardia occurred, the researchers recorded them as adverse events. If systolic blood pressure (SBP) declines by more than 30% compared to the preoperative SBP, or the SBP was less than 90 mmHg, the authors considered hypotension to have occurred; continuous doses of inotropes, e.g., epinephrine and dopamine were controlled, and ephedrine and calcium gluconate were administered. In addition, appropriate treatment such as intravenous fluid therapy were performed. When the heart rate was observed to be less than 50 beats per minute, bradycardia was considered to have occurred and was treated with inotropic agents.
Statistical analysis
All results were expressed as mean ± standard deviation, frequency (number or percentage of patients), or median (lowest value, highest value) depending on the nature of the data. The Student’s t-test was used to compare demographic data (age, height, weight etc.), operative and anesthetic time, and other continuous data. Pearson’s chi-square test or Fisher’s exact test was used to compare the frequencies of delirium, shivering, nausea, vomiting, the use of additional analgesics, and the use of inotropes. Continuous measurements of blood pressure, pulse rate, pulmonary artery pressure, and cardiac index, were measured after the administration of the test drug and the control drug, and were analyzed by repeated measures using analysis of variance (ANOVA) and Bonferroni correction, to compare differences between groups (multiple comparative test). For the primary outcome of RASS, the two groups were compared with the two-tailed, unpaired t-test with Welch’s correction.
A statistical analysis was performed using GraphPad Prism for Windows (version 6.00, GraphPad Software, USA), and R for Windows version 3.0.0 (The R Foundation for Statistical Computing, Austria). Results were statistically significant at P < 0.05.
The data of RASS, VAS, CI, blood pressure, heart rate, and pulmonary arterial pressure were recorded every two hours and data from 14 hours after ICU arrival with a few missing data were used for data analysis. For the sample size matching between groups, only the data of 19 subjects in each group were used.
Go to :

RESULTS
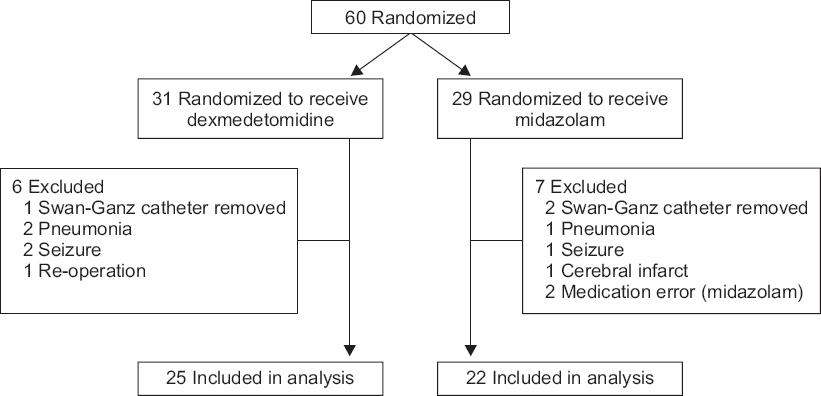
A total of 60 patients participated in the study: 31 in the DEX group and 29 in the MDZ group. Each test drug was administered according to the order of given subjects, and 47 of them were statistically analyzed: 25 in the DEX group and 22 in the MDZ group (
Fig. 1). Thirteen of the randomized patients were excluded from the analysis. Swan-Ganz catheter was removed and pulmonary artery pressure was not measured (n = 3). Ventilator weaning was difficult due to pneumonia (n = 3). Lorazepam and vecuronium were administered due to seizure (n = 3). Rt. MCA infarction occurred (n = 1). A Midazolam injection was not performed as protocol (n = 2). Re-operation for bleeding control (n = 1). There was no statistically significant difference in preoperative ejection fraction, initial cardiac index and intraoperative bleeding of study population (
Table 1).
 | Fig. 1
|
Table 1
Ejection Fraction, Bleeding, Cardiac Index and Transfusion
|
Variable |
MDZ (n = 22) |
DEX (n = 25) |
P value |
|
Preoperative EF (%) |
51.9 ± 15.5 |
49.6 ± 11.8 |
0.561 |
|
Postoperative EF (%) |
51.8 ± 12.5 |
50.5 ± 9.0 |
0.668 |
|
Initial CI (L/min/m2)*
|
2.5 ± 0.4 |
2.6 ± 0.6 |
0.532 |
|
Intraoperative blood loss (ml) |
1,509.1 ± 702.3 |
1,584.0 ± 583.6 |
0.692 |
|
Postoperative blood loss (ml) |
599.5 ± 431.7 |
522.8 ± 295.8 |
0.476 |
|
Intraoperative transfusion of RBC product, total amount (ml) |
1,365.9 ± 462.3 |
1,536.8 ± 597.6 |
0.283 |
|
Postoperative transfusion of RBC product, total amount (ml) |
394.5 ± 459.8 |
364.0 ± 384.4 |
0.805 |

The mean of the fraction within the target sedation level in each patient’s total sedation time was higher in the DEX group: 41.0% for dexmedetomidine-treated patients and 20.7% for midazolam-treated patients (P = 0.026), 95% confidence interval of difference between means (0.022 to 0.384). RASS was significantly lower in the DEX group than RASS in the MDZ group (P < 0.001). Both time and type of medication contributed to the difference (
Fig. 2). There was no difference between the two groups in the degree of pain (VAS) and the amount of additional pain control (
Table 2).
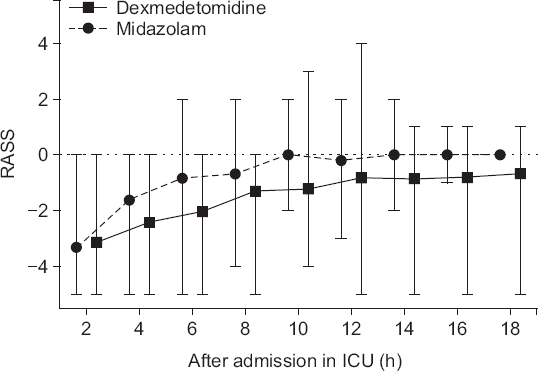 | Fig. 2Postoperative Richmond Agitation-Sedation Scale (RASS). RASS was significantly lower in the DEX group than that in the MDZ group (P < 0.001). Solid line presents median of dexmedetomidine. Dashed line presents median of midazolam. Error bars are expressed as lowest and highest value, each. DEX: dexmedetomidine, MDZ: midazolam, ICU: intensive care unit. 
|
Table 2
Postoperative Outcomes of Study Population
|
Variable |
MDZ (n = 22) |
DEX (n = 25) |
P value |
|
Delirium |
2 (9.1) |
1 (4.0) |
0.909 |
|
Vasopressor use |
20 (90.9) |
21 (84.0) |
0.787 |
|
Convert to PSV mode (min) |
832.0 ± 273.1 |
970.2 ± 418.4 |
0.194 |
|
Weaning time (min) |
957.3 ± 266.1 |
1,069.9 ± 430.2 |
0.281 |
|
LOH (d) |
12.2 ± 4.2 |
13.6 ± 5.8 |
0.380 |
|
Hypotension*
|
1.0 ± 1.3 |
1.3 ± 1.5 |
0.495 |
|
Bradycardia†
|
0 (0.0) |
1 (4.0) |
1.000 |
|
Desaturation‡
|
0 (0.0) |
0 (0.0) |
0.662 |
|
Vomiting (n) |
|
|
|
|
1 |
2 (9.1) |
2 (8.0) |
0.986 |
|
2 |
1 (4.5) |
1 (4.0) |
|
|
Additional sedation§
|
|
|
|
|
No |
16 |
18 |
0.608 |
|
Yes |
6 |
7 |
|
|
Fentanyl use‖
|
|
|
|
|
No |
22 (100.0) |
21 (84.0) |
0.112 |
|
Yes |
0 (0.0) |
4 (16.0) |
|
|
VAS for pain |
|
|
|
|
≤ 4 |
19 |
21 |
0.575 |
|
5 ≤ |
3 |
4 |
|
|
ICU length of stay (d) |
|
|
|
|
1 |
0 (0.0) |
0 (0.0) |
0.235 |
|
2 |
0 (0.0) |
2 (8.0) |
|
|
3 |
21 (95.5) |
19 (76.0) |
|
|
4 |
1 (4.5) |
2 (8.0) |
|
|
6 |
0 (0.0) |
2 (8.0) |
|

There was no significant difference in the preoperative EF and CI before cardiopulmonary bypass between the two groups. Postoperative CI was significantly lower in the DEX group than in the MDZ group (P = 0.047). However, there was no difference in postoperative EF between the two groups. Hemodynamic parameters and other variables were not significant between the two groups in the repeated measures ANOVA analysis (
Table 2). There was no statistically significant difference in the frequency of patients complaining of moderate to severe pain (VAS for pain score of 5 points or more) between the two groups in the Fisher’s exact test (
Table 2). There was no difference in the amount of bleeding and the amount of transfusion during and after surgery (
Table 2). One incident of bradycardia occurred in the MDZ group, while no incidents occurred in the DEX group (
Table 2).
Go to :

DISCUSSION
It is important to set the appropriate level of sedation for patients who are admitted to the ICU after cardiac surgery. Dexmedetomidine and midazolam are commonly used medications for sedation in the ICU [
8]. Dexmedetomidine has low respiratory depressive effects and the patient is easily arousable during sedation, allowing easy confirmation of their conscious state [
4]. However, it can cause bradycardia and cardiac depression [
9,
10].
Midazolam is a benzodiazepine-based drug that acts on the central nervous system and produces sedative and anxiolytic effects. It can decrease blood pressure and pulse rate and suppresses respiration when an excessive intravenous injection is administered too quickly [
11,
12].
In a study by Riker et al. [
6], there was no difference in the percentage of time within the target RASS range between dexmedetomidine and midazolam when both drugs were infused continuously. However, in this study, the percentage of time within the target level of sedation (RASS score, −2 to −1), during the sedation period of all patients was higher in the DEX group. In addition, the mean of the fraction that reached the target sedation level in each patient’s total sedation time was higher in the DEX group. This implies that dexmedetomidine continuous infusion is more effective in managing sedation levels than intermittent midazolam administration. Considering the characteristics of dexmedetomidine, which is characterized by low respiratory depression and allows easy termination of mechanical respiration [
4], deep sedation was easier to maintain in the DEX group than in the MDZ group because it was possible to administer a sufficient amount of drug to maintain deep sedation (
Fig. 2). The dexmedetomidine group maintained a relatively deep sedation, with no evidence of associated hemodynamic change (
Fig. 3).
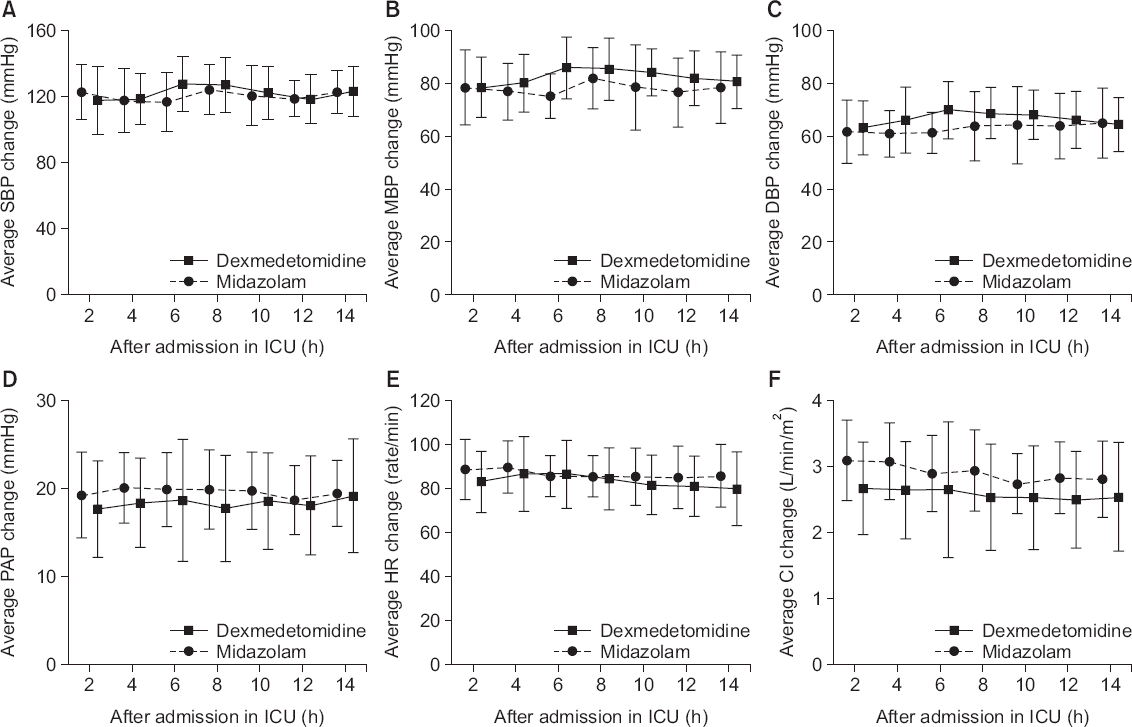 | Fig. 3Postoperative hemodynamic factors. Solid line presents mean of dexmedetomidine. Dashed line presents mean of midazolam. Error bars are expressed as standard deviation. (A) Average of systolic blood pressure (SBP), (B) average of mean blood pressure (MBP), (C) average of diastolic blood pressure (DBP), (D) average of pulmonary arterial pressure (PAP), (E) average of heart rate (HR), (F) average of cardiac index (CI). The DEX group had a relatively low CI compared to the MDZ group, and there was no significant difference in other hemodynamic parameters. DEX: dexmedetomidine, MDZ: midazolam, ICU: intensive care unit. 
|
Dexmedetomidine is known to have analgesic and opioid-sparing effects [
4] and has been reported to have the same effect in major surgery, such as cardiac surgery [
13]. This study showed no differences in VAS for pain and the amount of additional pain control between groups.
A loading dose according to the recommended administration method of dexmedetomidine may cause systemic vasoconstriction, hypertension [
14], excessive sedation and airway obstruction [
15], and a high maintenance dose (> 0.7 μg/kg/h) may cause bradycardia [
16]. In the present study, the loading dose of dexmedetomidine was omitted and titration was continued with less than 0.7 μg/kg/h.
Dexmedetomidine is known to be associated with bradycardia [
10] and the reported decrease in cardiac function is due to bradycardia, rather than a decrease in cardiac output [
17]. There is a relationship between the dose of dexmedetomidine and the decrease in cardiac function [
9,
10]. For this reason, caution should be exercised when using dexmedetomidine in the presence of impaired cardiac function, including bradycardia or atrioventricular nodal block [
18,
19]. In this study, the DEX group did not show a decrease in heart rate (
Fig. 3).
The DEX group had a relatively low CI compared to the MDZ group, but in both groups, mean CI was within the normal range (MDZ, 2.742–3.095; DEX, 2.542–2.753). There was no significant difference in hemodynamic changes (
Fig. 3). In addition, there was no significant difference in preoperative and postoperative EF between two groups. Therefore, the decrease of CI is transient and does not have a clinical effect on future cardiac function and clinical prognosis. However, when dexmedetomidine is given to patients with impaired cardiac function, it should be used with caution as indicated in various reports [
19,
20]. Dexmedetomidine has been reported to be effective in reducing the use of antiemetics and the length of intensive care unit stay and general hospital stay [
16,
21], but there was no statistically significant difference in this study.
Hypotension after cardiac surgery is very frequent and has a significant effect on mortality and postoperative prognosis. Although hypotension is one of the important side effects of dexmedetomidine [
4], there was no statistically significant difference in this study (
Fig. 3). This study was a prospective, randomized controlled, open-label, parallel group design clinical study. The scale of the research was small, but the number of patients ruled out by the exclusion criteria was large. Post-hoc power analysis was carried out. The power was 60.9%. There is a risk of inducing type II error due to low power. If more clinical trials are conducted including more patients, there will be more meaningful results in the future. There was a difference between the medication injection method of the two groups according to the conventional dosing regimen, i.e. intermittent midazolam administration and continuous infusion of dexmedetomidine, which led to an open-label study rather than a double-blind study. Drug administration methods may also have affected the outcome of the study.
In conclusion, this study showed that post-operative infusion of dexmedetomidine maintained a stable sedation. But, this study has a low post-hoc power. There is a risk of inducing type II error due to low power. Therefore, the researchers cannot conclude that dexmedetomidine is better sedative than midazolam. If more clinical trials are performed on more patients, there will be more meaningful results. There was no difference in postoperative clinical course and prognosis between the two groups. Careful use will be required for patients with severe cardiac dysfunction because the CI was low although within the normal range.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download