INTRODUCTION
Rocuronium has generally been used in anesthetic practice by bolus injection for muscle relaxation during tracheal intubation or by continuous infusion [
1]. However, rocuronium may induce a withdrawal response due to intense injection pain, which is characterized by considerable movement during injection, with an overall incidence of about 80% (range, 56%–100%) [
1–
3].
Many clinical studies have tried to reduce the withdrawal response by various pharmacological interventions [
2–
10]. Most reported that lidocaine and remifentanil were effective in reducing the incidence of rocuronium-induced withdrawal response [
1,
6,
9]. Some authors suggested that non-opioid analgesics such as tramadol and paracetamol also effectively reduced rocuronium-induced withdrawal response, even though their effects were less than that of lidocaine [
4,
6]. In addition, serotonin receptor antagonists such as ondansetron and palonosetron were also effective in the prevention of rocuronium-induced withdrawal response, although there is some controversy regarding the effectiveness of serotonin receptor antagonists compared to that of lidocaine [
5–
8].
In the clinical setting, however, we frequently encounter patients with many kinds of analgesic (such as nonsteroidal anti-inflammatory drugs, nefopam or opioids) infusions or single bolus injections to control disease-related pain prior to anesthesia. We cannot ignore the fact that preoperative treatment with these analgesics influences rocuronium-induced withdrawal response. Nefopam (a non-narcotic, nonsteroidal, centrally acting analgesic) is usually used for the treatment of nociceptive pain and the prevention of postoperative shivering and hiccups with a similar mechanism to that of serotonin, norepinephrine, and dopamine reuptake inhibitors, although the mechanism of nefopam has not been clearly demonstrated [
11]. In critically ill patients with moderate-to-severe pain, nefopam is also reported to be an effective alternative to opioids without significant changes in Richmond Agitation Sedation Scale score, ventilatory frequency, or oxygen saturation [
12]. Thus, we may encounter patients with nefopam infusion prior to anesthesia. However, to our knowledge, no study has assessed the effect of nefopam on rocuronium-induced withdrawal response. In particular, the synergistic or additional effects of remifentanil on the incidence of rocuronium-induced withdrawal response must be considered in total intravenous anesthesia with propofol and remifentanil. Therefore, we hypothesized that nefopam would be effective in preventing rocuronium-induced withdrawal response by synergistic or additional effects with remifentanil.
In the present study, we investigated the effect of nefopam on rocuronium-induced withdrawal response in patients undergoing elective surgery under total intravenous anesthesia with propofol and remifentanil in a clinical setting. We recorded the presence of rocuronium-induced withdrawal response (primary outcome). We also monitored invasive blood pressure, heart rate, and respiratory rate (secondary outcomes) after the injection of rocuronium as well as nefopam-associated side effects.
MATERIALS AND METHODS
We registered this study in the Clinical Research Information Service (CRIS:
https://cris.nih.go.kr/) on June 27, 2016. The registration number is ‘KCT0001959’.
This prospective, randomized, controlled, double blinded study was approved by the Institutional Review Board of School of Medicine, Chosun University (No. CHOSUN 2016-02-001-001). After obtaining written informed consent from all patients or their guardians, we enrolled 76 patients aged 20 to 65 years with American Society of Anesthesiologists physical status classification of I or II who were scheduled to undergo elective surgery under total intravenous anesthesia. We excluded patients with glaucoma, neuromuscular disease, hepatic or renal function abnormality, convulsive disorder, mental disorder, and moderate-to-severe cardiovascular diseases. We excluded patients who could not access the intravenous catheter on the dorsum of their hands, and who had an allergy to the study drug, medication such as anticonvulsants, antidepressants, or opioids. We also excluded patients at risk of urinary retention, pregnant women, breastfeeding women, or women planned to become pregnant. The patients and investigators were blinded to the study medications; a nurse randomized medications to be indistinguishable and numbered syringes using a table of random numbers.
All patients were randomly allocated into one of two groups using the random number table (
Fig. 1). In the control group (n = 38), 102 ml of 0.9% sodium chloride solution was infused one hour before surgery at a rate of 100 ml/h. In the nefopam group (n = 38), 20 mg nefopam (2 ml) in 100 ml of 0.9% sodium chloride solution was infused one hour before surgery at a rate of 100 ml/h (
Fig. 2). All patients were premedicated with 0.05 mg/kg intramuscular midazolam 30 minutes before anesthesia induction. Standard monitoring included an electrocardiogram, non-invasive blood pressure, end-tidal partial pressure of carbon dioxide, and peripheral pulse oximetry. Anesthesia was induced with remifentanil and propofol at target effect-site concentrations of 2.0 ng/ml and 3.0 μg/ml with a target-controlled infusion (TCI) pump (Orchestra
®, Fresenius Vial, France). One minute after equilibration of the target plasma and effect-site concentrations of propofol and remifentanil, rocuronium (0.6 mg/kg) was injected over 5 to 10 seconds, and the grades of rocuronium-induced withdrawal response on a four-point scale (0: no response, 1: wrist withdrawal, 2: arm only, 3: generalized movement) were assessed before intubation [
9]. We defined moderate to severe withdrawal response using grade > 1 as the cutoff for significant rocuronium-induced withdrawal response. We monitored the invasive arterial blood pressures, heart rate, and respiratory rate upon operating room arrival, after anesthesia induction, and one minute after rocuronium injection. Patient age, sex, American Society of Anesthesiologists physical status classification, height, weight, and nefopam-associated side effects (including sedation, dry mouth, tachycardia, dizziness, sweating, nausea and vomiting, dysphoria, diplopia, and dizziness) were also noted. All patients were transferred to the recovery room after restoration from a neuromuscular block with reversal agents (pyridostigmine 0.15 mg/kg with glycopyrrolate 0.2 mg/5 mg of pyridostigmine) at the end of surgery.
Fig. 1
CONSORT flow chart. Control group received 102 ml of 0.9% sodium chloride solution and nefopam group received 20 mg nefopam (2 ml) in 100 ml of 0.9% sodium chloride solution, one hour before surgery at a rate of 100 ml/h. Anesthesia was induced with remifentanil and propofol with a target-controlled infusion pump.
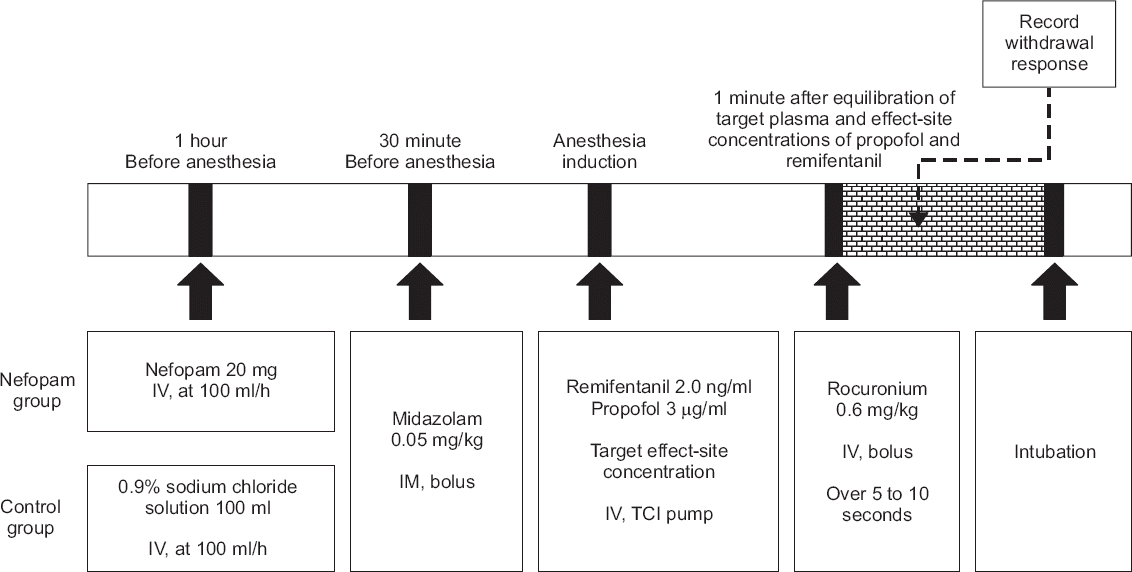
Fig. 2
Study schematic protocol. Control group received 102 ml of 0.9% sodium chloride solution and nefopam group received 20 mg nefopam (2 ml) in 100 ml of 0.9% sodium chloride solution one hour before surgery at a rate of 100 ml/h. Anesthesia was induced with remifentanil and propofol with a target-controlled infusion (TCI) pump. Rocuronium (0.6 mg/kg) was then injected at one minute after the equilibration of the target plasma and effect-site concentrations of propofol and remifentanil. IV: intravenous, IM: intramuscular.
Statistical analysis
The appropriate sample size was calculated by defining the level of statistical significance as α = 0.05 and β = 0.2 using z-tests of G*Power software (
www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3, Germany, ver. 3.1.9.2) with the expected proportions of incidence of withdrawal response in control group (50%) and in nefopam group (20%) [
13,
14], because there was no evidence for calculating the effect size on the effect of nefopam and remifentanil combination. We required a total of 76 patients, with 38 patients in each group without a dropout rate.
IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., USA) was used for statistical analysis. All measured values are presented as means ± standard deviation, means (95% confidential intervals) or numbers and percentages of patients (n [%]). The incidence and grades of rocuronium-induced withdrawal response, sex, American Society of Anesthesiologists physical status classification, and nefopam-associated side effects were analyzed by chi-squared tests for normally distributed data. The invasive arterial blood pressures, heart rate, respiratory rate, age, and weight were analyzed by Student’s t-tests for normally distributed data. A P value < 0.05 was considered to indicate statistical significance.
RESULTS
Seventy-six patients were enrolled, but two patients in nefopam group were dropped from the statistical analysis because of incomplete infusion and refusal due to nausea and cold sweats during the nefopam infusion (
Fig. 1).
There were no significant differences in the age, sex, height, weight, and American Society of Anesthesiologists physical status classification between control group and nefopam group (
Table 1).
Table 1
|
Characteristic |
Control group (n = 38) |
Nefopam group (n = 36) |
P value |
|
Sex (M/F) |
17/21 |
11/25 |
0.209 |
|
ASA (I/II) |
29/9 |
28/8 |
0.881 |
|
Age (yr) |
42.6 ± 13.3 |
41.5 ± 12.1 |
0.717 |
|
Height (cm) |
165.6 ± 10.0 |
162.8 ± 7.7 |
0.176 |
|
Weight (kg) |
66.6 ± 12.9 |
62.9 ± 10.7 |
0.183 |
The overall incidence of rocuronium-induced withdrawal response was significantly lower in nefopam group (27.8%, n = 36) than in control group (60.5%, n = 38) (P = 0.005,
Table 2). The incidence of rocuronium-induced withdrawal response differed significantly between nefopam group and control group (P = 0.017,
Table 2). The incidence of moderate to severe withdrawal response, 19.4%, was also lower in nefopam group (n = 36) than that in control group (42.1%, n = 38) (P = 0.035,
Table 2).
Table 2
Incidence and Grades of Rocuronium-induced Withdrawal Responses
|
Variable |
Control group (n = 38) |
Nefopam group (n = 36) |
P value |
|
Overall incidence of withdrawal response |
23 (60.5) |
10 (27.8) |
0.005 |
|
Moderate to severe withdrawal response |
16 (42.1) |
7 (19.4) |
0.035 |
|
Grade of withdrawal response |
|
|
0.017 |
|
0: no response |
15 (39.5) |
26 (72.2) |
|
|
1: wrist withdrawal |
7 (18.4) |
3 (8.3) |
|
|
2: arm only |
8 (21.1) |
6 (16.7) |
|
|
3: generalized movement |
8 (21.1) |
1 (2.8) |
|
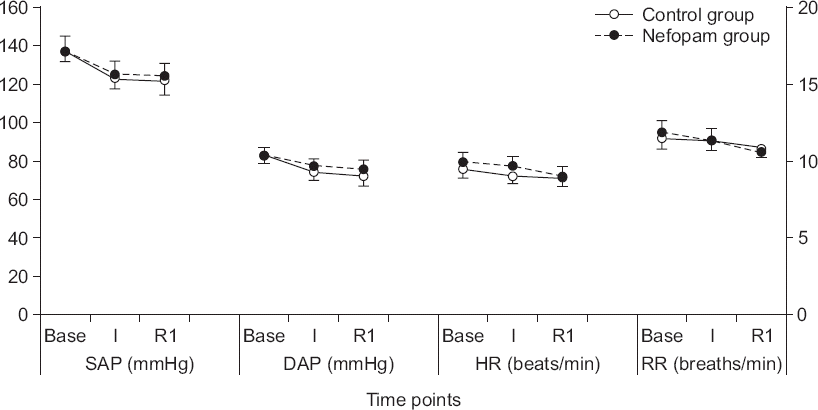
There were no significant differences in arterial pressure, heart rate, and respiratory rate between control group and nefopam group (
Fig. 3).
Fig. 3
Arterial pressures (systolic and diastolic), heart rate, and respiratory rate. There were no significant differences between groups. Control group received 102 ml of 0.9% sodium chloride solution and nefopam group received 20 mg nefopam (2 ml) in 100 ml of 0.9% sodium chloride solution one hour before surgery at the rate of 100 ml/h. The right Y axis indicates arterial pressures (systolic and diastolic) and heart rate, while the left Y axis indicates the respiratory rate. Base: values upon operating room arrival, I: values after anesthesia induction, R1: values at one minute post-injection of rocuronium, SAP: systolic arterial pressure, DAP: diastolic arterial pressure, HR: heart rate, RR: respiratory rate.
DISCUSSION
In the present study, the additional pretreatment with nefopam (20 mg) one hour before induction showed a significantly lower incidence (27.8%) of withdrawal response after the injection of rocuronium than that observed with remifentanil alone at a target effect-site concentration of 2.0 ng/ml (60.5%).
Most previous studies reported that single bolus doses of remifentanil (0.5 or 1 μg/kg) were effective in reducing the incidence of rocuronium-induced withdrawal response to 0% to 6.3% [
1,
9,
15,
16]. Instead of a single injection, we can also administer remifentanil using a TCI pump. For TCI, some authors calculated the target effect-site concentration of remifentanil with a 50% probability of preventing rocuronium-induced withdrawal response (EC
50) using Dixon’s up-and-down method [
13,
17]. They reported rocuronium-induced withdrawal responses using the four-grade system utilized in the present study, in which a grade of 2 or more was regarded as a significant (moderate-to-severe) response. Park et al. [
13] reported that the EC
50 of remifentanil were 1.8 ± 0.5 and 2.3 ± 1.0 ng/ml, respectively, in male and female patients between 20 and 60 years of age [
13]. Yoon et al. [
17] documented a remifentanil EC
50 of 1.37 ng/ml and EC
95 of 3.19 ng/ml during the infusion of propofol at a target effect-site concentration of 3 μg/ml [
17]. In this study, our interventional time design of drugs (remifentanil, propofol, and rocuronium) was same as that of Yoon et al.’s study [
17], in which remifentanil and propofol were simultaneously infused using a TCI pump and rocuronium was injected after equilibration of the target plasma and effect-site concentrations of propofol and remifentanil. In addition, they also utilized a four-point scale, with grade > 1 defined as moderate to severe withdrawal response, as in the present study. Therefore, the 42.1% incidence of moderate to severe rocuronium-induced withdrawal response is not surprising because we infused remifentanil at a target effect-site concentration of 2.0 ng/ml, a dose between the EC
50 and EC
95 reported in Yoon et al.’s study [
17].
Single (1 μg/kg) or continuous infusion of remifentanil may induce significant frequent coughing, hypotension, and bradycardia as well as difficult ventilation due to chest tightness and muscle rigidity, resulting in desaturation < 90% [
15,
16]. Therefore, because these side effects frequently occur when opioids are administered rapidly, a slow infusion is recommended for bolus doses of opioids [
16]. Based on this concept, in our hospital, we usually slowly administer remifentanil in a single bolus or start to infuse remifentanil at target effect-site concentration of 2.0 ng/ml, increasing in steps of 0.5 ng/ml according to the presence of side effects. Thus, we fixed the infusion rate of remifentanil at a target effect-site concentration of 2.0 ng/ml in order to minimize remifentanil-related side effects.
Nefopam (a non-narcotic, nonsteroidal, centrally acting analgesic) likely has a similar mechanism of action as that of serotonin, norepinephrine, and dopamine reuptake inhibitors, although its precise mechanism has not been clearly elucidated [
11,
18,
19]. Studies of serotonin receptor antagonists reported that ondansetron and palonosetron were effective in the prevention of rocuronium-induced withdrawal response [
5–
8]. The overall incidences of rocuronium-induced withdrawal response were 38% to 45% in palonosetron (0.075 mg), 44% in ondansetron (4 mg), and 87.5% to 75% in 0.9% isotonic saline groups [
5,
6,
8]. The incidences of moderate to severe withdrawal response were 8% to 22.5% in palonosetron, 16% in ondansetron (4 mg), and 58% to 65% in 0.9% isotonic saline groups [
5,
6,
8]. Unfortunately, we did not investigate the incidence of rocuronium-induced withdrawal response in patients who received nefopam pretreatment alone. Therefore, we cannot determine whether pretreatment with nefopam is as effective as that with serotonin receptor antagonists in reducing the incidence of withdrawal response. Just, we can assume that the overall incidence of rocuronium-induced withdrawal response in nefopam pretreatment may be similar to that observed in serotonin receptor antagonist pretreatment.
In this study, the overall incidence of rocuronium-induced withdrawal response was lower (27.8%) in the group that received a continuous infusion of remifentanil after nefopam pretreatment compared with those (38% to 45%) in groups that received serotonin receptor antagonists [
5,
6,
8]. The incidence was reduced by approximately 30% more in the group that received a continuous infusion of remifentanil after nefopam pretreatment than that during remifentanil infusion alone (60.5%). This discrepancy may be explained by a synergistic or additional effect between nefopam and remifentanil.
Nefopam has several potential adverse events, including sedation, dry mouth, tachycardia, dizziness, sweating, nausea and vomiting, dysphoria, diplopia, and dizziness [
11,
20–
23]. Therefore, the manufacturer recommends injecting nefopam slowly in order to prevent these adverse events. Even though tachycardia and profuse sweating occurred more frequently, many studies reported that these adverse events associated with nefopam were not observed or were minor. In addition, nefopam was recently reported to cause frequent injection pain [
24]. Kim et al. [
24] showed that the incidence of injection pain was lower in patients infused with 30 mg nefopam at 60 ml/h (over 20 minutes), compared with that at a rate greater than 120 ml/h (below 10 minutes). Kim and Abdi [
11] also suggested that intravenous nefopam should be slowly infused in single doses of 20 mg over 15 to 20 minutes to minimize adverse effects during treatment of neuropathic pain. Chanques et al. [
12] reported that the onset time of nefopam (20 mg) was at least 15 to 30 minutes and that its peak effect occurred at least 30 to 60 minutes after the beginning of the infusion. In this context, we initially designed this study at a rate of 200 ml/h but received the pharmaceutical recommendation that a rate of 100 ml/h would be safer than that of 200 ml/h to minimize the incidence of side effects. Therefore, we infused 20 mg nefopam in 100 ml of 0.9% sodium chloride solution at a rate of 100 ml/h one hour before surgery in order to minimize the adverse events of nefopam. In this study, one patient complained of nausea and cold sweats, which subsided after stopping the nefopam infusion. In addition, there was no injection pain or significant hemodynamic/respiratory adverse events.
There are some limitations associated with the present study. First, we did not evaluate the incidence of rocuronium-associated withdrawal responses using 0.9% sodium chloride solution. It was not necessary to assess the incidence of withdrawal response in the control group administered 0.9% sodium chloride solution because many studies have already reported an incidence above 80% [
5,
6,
8]. Second, we did not evaluate the incidence of rocuronium-induced withdrawal responses using nefopam alone. Evaluation of this group may be necessary to compare the effect on the reduction of withdrawal response with remifentanil alone and the remifentanil-nefopam combination. Third, we did not clarify the mechanism by which nefopam reduces the incidence of rocuronium-induced withdrawal response. Fourth, nefopam may influence the neuromuscular block because it was initially developed as a muscle relaxant, even though we did not experience any significantly delayed recovery from the neuromuscular block and postoperative residual block in this study [
11,
25,
26]. Based on these limitations, further study is required to reveal whether nefopam has local or central effects on the pain control mechanism, whether it has synergistic or additional effects with opioids, and whether it may also influence the effect of neuromuscular blockers. Finally, the sample size of this study was calculated without a dropout rate and two patients were excluded from the analysis. Thus, this study did not satisfy the initial assumed power (0.8).
In conclusion, the continuous infusion of remifentanil after nefopam pretreatment (20 mg) one hour before the induction of anesthesia was more effective in terms of reducing the incidence of rocuronium-induced withdrawal response with stable hemodynamics than the continuous infusion of remifentanil alone at a target effect-site concentration of 2.0 ng/ml. This finding may be helpful in additional studies to determine if preoperative treatments with other analgesics to control disease-related pain influence rocuronium-related withdrawal responses.







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download