INTRODUCTION
The use of a neuromuscular blocker (NMB) is often required for general anesthesia, and various types of NMBs are currently in use. A sufficient depth of neuromuscular block can facilitate surgical approach; however, postoperative residual neuromuscular blockade (PRNB) may also occur, resulting in delayed recovery, aspiration pneumonia, and hypoxia due to apnea. If PRNB persists, it may lead to fatality results caused by airway obstruction, hypoxia, and hypercapnia [
1]. The use of long-acting NMBs such as pancuronium is known to increase the incidence of PRNB [
2]. However, many researchers reported that NMB with moderate duration of action such as rocuronium, vecuronium, and atracurium could cause to PRNB [
3,
4].
We can objectively measure the depth of neuromuscular block by utilizing neuromuscular monitoring during surgery, which can reduce PRNB [
5]. Previously, Seo et al. [
6] reported in a survey of Korean anesthesiologists that the respondents were interested in preventing PRNB; yet the utilization rate of neuromuscular monitoring was still low. Most responders (85.9%) considered PRNB to be a serious problem. Nevertheless, they preferred clinical evaluation (83.3%) to neuromuscular monitoring. Not all hospitals have sufficient quantitative neuromuscular monitoring devices.
We conducted a cross-sectional nationwide survey to evaluate the attitudes, knowledge and practice of Korean anesthesiologists in regards to the use of neuromuscular blocking drugs and antagonists. We also surveyed the same aspects regarding neuromuscular monitoring. We compared our results with those from previous studies in Korea as well as abroad.
MATERIALS AND METHODS
The survey was conducted from 2013 to 2014. After approval by the board of directors in the Korean Society of Anesthesiologists, we explained the purpose of this investigation by sending e-mails to a total 3,736 regular members of the Korean Society of Anesthesiologists with registered e-mail addresses. Upon consenting to take part in the survey, they downloaded the questionnaire and returned it by e-mail. The response rate of the university hospitals, clinics, and general hospitals except for the outpatient clinic was 23.7%.
The questionnaire consisted of 39 items (7 items for demographic data and 32 items for NMB and monitoring). We divided the questionnaire into several points; 1) current concept about PRNB, 2) current practice of NMBs and its antagonists, 3) how to assess recovery from neuromuscular block, and 4) availability and use of neuromuscular monitoring equipment by each institution.
RESULTS
Demographic results
We received replies submitted to the questionnaire from 86 anesthesiologists with an overall response rate of 23.7%. The respondents belonged to university hospitals, clinics, and general hospitals, excluding outpatient clinics. Of these 86 respondents, 61 (70.9%) had completed residency training five or more years ago. Sixty-two (72.1%) of the respondents were practicing at the university hospital or an affiliate. On the other hand, 12 (14.0%) of the respondents were affiliated with non-teaching general hospitals and the others (14.0%) were local clinic practitioners (
Table 1).
Table 1
|
Question |
Result (n = 86) |
|
Years of professional experience after resident training |
|
|
1–5 |
25 (29.1) |
|
6–10 |
20 (23.3) |
|
11–15 |
12 (14.0) |
|
16–20 |
15 (17.4) |
|
> 20 |
14 (16.3) |
|
Type of hospital |
|
|
University hospital |
58 (67.4) |
|
Affiliated university hospital |
4 (4.7) |
|
General hospital (nonteaching) |
12 (14.0) |
|
Local clinics (practitioner) |
12 (14.0) |
Concepts of postoperative residual neuromuscular blockade
In regards to PRNB, 87.2% of the respondents replied that they had experience with patients who had respiratory obstruction, re-intubation, dyspnea, hypoxia, etc. in the recovery room after surgery due to PRNB. The response rate of PRNM was less than 1% was 64%. On the other hand, the response of 19.8% answered that the incidence was less than 5%, the response of 74.4% indicated that the routine use of neuromuscular monitoring could decrease the incidence of PRNB (
Table 2).
Table 2
Attitude and Practice of Postoperative Residual Neuromuscular Blockade (PRNB)
|
Question |
Result (n = 86) |
|
Do you experience PRNB in post anesthetic care unit? |
|
|
Yes |
11 (12.8) |
|
No |
75 (87.2) |
|
What is the incidence (%)of clinically significant PRNB in your hospital? |
|
|
< 1 |
55 (64.0) |
|
1–5 |
17 (19.8) |
|
6–20 |
0 |
|
> 20 |
0 |
|
I don’t know |
14 (16.3) |
|
Do you think PRNB cause significant complications? |
|
|
Yes |
9 (10.5) |
|
No |
77 (89.5) |
|
Do you think routinely using neuromuscular monitoring can reduce PRNB? |
|
|
Yes |
64 (74.4) |
|
No |
1 (1.7) |
|
No response |
21 (24.4) |
Practice of muscle relaxants and their reversal
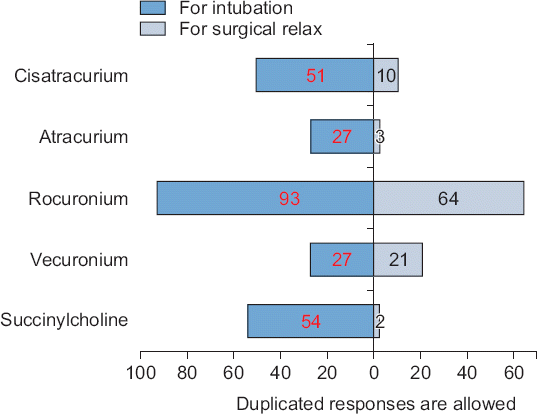
The following questions contains of multiple answers. The availability of NMBs in the operating room in decreasing order was as follows: rocuronium (93.0%), succinylcholine (87.2%), vecuronium (82.6%), cisatracurium (66.3%), atracurium (41.9%), and pancuronium (1.2%) (
Fig. 1). Rocuronium (92.9%) was the most common NMB used in tracheal intubation, followed by succinylcholine (54.1%), cisatracurium (50.6%), vecuronium (27.1%), and atracurium (27.1%). Rocuronium (64.4%) was the most commonly used NMB during surgery, followed by vecuronium (20.6%), cisatracurium (10.4%), atracurium (2.5%), and succinylcholine (2.2%) (
Fig. 1).
Fig. 1
Most often daily used neuromuscular blockers. This figure shows the data on the use of various neuromuscular blockers for tracheal intubation and surgical relaxation. Most of the respondents prefer to use a rocuronium for tracheal intubation and surgical relaxation. More than half of respondents still use succinylcholine for tracheal intubation.
The use of an anticholinesterase as antagonist, at the end of the operation when using a non-depolarizing NMB was 94.2%. Of the respondents who indicated that they might skip the antagonist, 60.0% of the respondents said they would skip 25.0% or less. The most common reasons for omitting the antagonist was the long duration of last dose of NMB (60.0%), the relatively high degree of train-of-four ratio (TOF, 50.0%), no need for antagonist because of near-complete recovery from relaxation (30.0%), small amount of total dose of NMB use (20.0%), and no fade (20.0%) when stimulated with simple peripheral nerve.
The predominant antagonists were pyridostigmine (81.2%) and neostigmine (22.4%). Among the respondents, for neostigmine, 40.0% used a dose of 0.05 mg/kg, 40.0% used less than 0.05 mg/kg, and 20.0% used a single dose of 2.5 mg regardless of the weight of the patient. For pyridostigmine, 39.4% of the respondents used a single dose of 10 mg, 38.0% used 0.25 mg/kg, 15.5% used less than 0.25 mg/kg, and 7.0% used more than 0.25 mg/kg. As regards the reason for using pyridostigmine, 79.2% of respondents answered that they used it because it has been in use for a long time. On the other hand, 9.7% replied because it gives better results and 4.2% replied for its reasonable price. Concerning the use of anticholinesterase and anticholinergic, 70.6% said they were concerned about the side effects, 71.6% said that side effects could be cardiovascular. Other side effects are nausea (27.0%), vomiting (16.2%), and related to the respiratory system (10.8%).
Determination of recovery from neuromuscular block
Of the total respondents, 84.7% said that a quantitative neuromuscular monitoring device was necessary and should be available in the operating rooms.
The response of 75.3% out of total answers said that clinical tests were a reliable indicator of recovery from a neuromuscular block, 37.4% of respondents thought there was no PRNB without any fade at 50 Hz tetanic stimulation.
In a question regarding when extubation would be possible after administration of neostigmine, the most frequent response was 3 to 5 minutes (42.9%), 6 to 10 minutes (28.6%), and more than 10 minutes (23.8%). Neostigmine was administered in the order of two (60.0%), four (25.0%) and three (10.0%) consecutive twitch responses after TOF stimulation. In a case of pyridostigmine, the time of extubation after administration of pyridostigmine was 3 to 5 minutes and 6 to 10 minutes, respectively, 32.9%, 11 to 15 minutes (20.0%), less than 2 minutes (8.6%), more than 15 minutes (5.7%). Pyridostigmine was administered in the order of four (40.6%), three (29.0%), two (21.7%) and one (4.4%) consecutive twitch response.
A total 42.2% of the respondents answered that a TOF-ratio more than 0.9 was required for extubation, followed by 0.8–0.9 (36.1%), 0.7–0.8 (15.7%), and 0.6–0.7 (1.2%). Five percent of the respondents said that the TOF-ratio is not important in determining extubation.
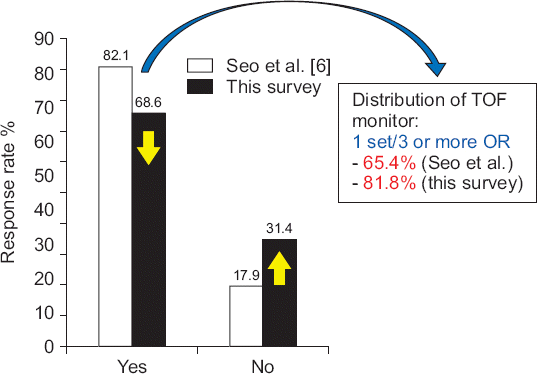
The availability and use of neuromuscular monitoring
Nevertheless, only 68.6% of the respondents were able to monitor the TOF-ratio using a quantitative peripheral nerve stimulator, By type, TOF-watch S
® or SX
® (Organon, Ireland) was the most common (74.1%), 29.3% for GE-nNMT
® (GE Healthcare, USA) and 6.9% for TOF-guard
® (Organon). In a question of the number of available monitor, the highest rate was 81.8% per 3 operating rooms, 10.9% per two, and 7.3% per one (
Fig. 2).
Fig. 2
The availability of quantitative neuromuscular monitoring in the operating room (OR). In comparison of previous survey (Seo et al. [
6]), the distribution rate decreased because the number of OR was increased rather than the supply of neuromuscular monitoring device over the years. TOF: train-of-four ratio.
More than 11.1% of respondents said that they use both of quantitative and simple peripheral nerve stimulator, but 24.4% said that they use neither of those.
DISCUSSION
This survey is the second nationwide survey on the use of NMBs with antagonists and neuromuscular monitoring in Korea. In 2008, Seo et al. [
6] had conducted the first survey using a questionnaire similar to that used by us. Their results were in many respects comparable to the results of our survey. A greater variety of NMBs are now available in the operating room than at the time of the previous survey. Particularly, the availability of cisatracurium has increased up to 66.3%. The official launching of cisatracurium in Korean market since 2010 caused the increased use of cisatracurium. In contrast, pancuronium is no longer available in the Korean market, as pharmaceutical companies have stopped manufacturing it because of the low profit margins. The respondents who answered that they could use pancuronium in the operating room (1 respondent, 1.2%) acquired it through stock drugs or other routes (direct foreign imports, etc.). However, they answered that they did not actually use a pancuronium for induction or maintenance of anesthesia.
In our survey, most of the respondents preferred to use rocuronium for tracheal intubation and surgical relaxation; this preference has not changed since the previous survey. More than half of the respondents still use succinylcholine for tracheal intubation, although the utilization rate has decreased from the previous survey. Traditionally, Korean anesthesiologists have preferred succinylcholine for a long time. We believe that the noticeable decline in the use of succinylcholine may due to the availability of sugammadex and increased awareness of the side effects of succinylcholine.
The use of pyridostigmine as an antagonist has decreased from 91% in 2008 to 81% at present, while that of neostigmine has increased from 15.4% to 22.4%. This was not significantly different from the previous results of Seo et al. [
6], who reported as 91% in pyridostigmine and 15.4% in neostigmine. These results are very different from those surveyed abroad. Eldawlatly and El-Tahan [
7] reported the use of neostigmine was 78%. In a study conducted by Naguib et al. [
8] in Europe and USA anesthesiologists, they did not mentioned the pyridostigmine as the question item for antagonists. Viby-Mogensen and Claudius [
9] also mentions neostigmine as the most preferred antagonist in the last 50 years because neostigmine has advantages over pharmacokinetic properties. The longer elimination half-life of pyridostigmine causes the longer duration of action compared with the others (neostigmine or edrophonium) [
10]. This implies that at the same time, it acts longer than the action time of the anticholinergics and increases the risk of side effects. Another advantage of neostigmine is that it is more potent (4.4 to 6.7 times) than pyridostigmine [
11]. Nevertheless, the preference for pyridostigmine is still high—a phenomenon unique to Korea. Survey respondents did not choose the sugammadex, one of the more popular antagonists in these days, because its use was not common at the time of the survey.
The number of quantitative peripheral nerve stimulators available for use has decreased compared to that at the time of the previous survey. The number of operating rooms in Korea has increased significantly over the years. However, the supply of neuromuscular monitoring devices has not kept up with the demand, and hence, the distribution rate has decreased.
More than 75% of the respondents responded positively to the usefulness of neuromuscular monitoring to reduce the incidence of PRNB. The rate of respondents who agreed that quantitative neuromuscular monitoring should be available in the operating room was 85%. Based on these results, we can conclude that most of the Korean anesthesiologists appreciate the usefulness of neuromuscular monitoring. However, although the trend has decreased since the last survey, more than 75% of the respondents still prefer to use clinical signs to evaluate the recovery from paralysis. They believe that clinical signs such as the ability to sustain a 5-second head lift are reliable indicators of adequate recovery from paralysis. However, these clinical tests do not completely predict PRNB [
12]. When the patient fails to perform these tests completely, the anesthesiologist cannot clearly distinguish between the state of PRNB and the effect of the sedative or anesthetic. On the other hand, many patients may be successful in performing these tests, even though they are in a condition that meets the criteria for PRNB (TOF-ratio < 0.7 or 0.9) [
13]. Ali et al. [
14] reported that a 5-second head-lift test could be performed even though the continuous stimulus ratio is 0.5. The causes of these problems are the high specificity, low sensitivity, low positive predictive value and negative predictive value. Another problem is that some degree of cooperation is required to perform clinical tests.
In order to ensure patient safety, the TOF-ratio should be recovered to more than 0.9 before extubation [
13]. In our survey, only about 40% of respondents selected this as the cut-off; more than 50% of the respondents accepted values less than 0.7 or 0.8 as adequate. Education and aggressive promotion at the institutional level are necessary to address this issue.
It is necessary to compare our findings with those of the survey by Naguib et al. [
8], which was the largest survey of NMBs conducted on European and American Anesthesiologists. In our survey, 89.5% of the respondents believed that PRNB could cause postoperative residual complications; the percentage was 75.2% in Europe and 77.7% in the United States, indicating a lower rate of awareness of the severity and potential risk of PRNB than in Korea. However, in Korea, only 74.4% of the respondents answered that the routine use of neuromuscular monitoring reduces the incidence of PRNB, in contrast to 85.7% in Europe and 80.0% in the United States. The distribution rate of nerve stimulators is higher in both Europe and the United States than in Korea, and it seems that they consider the routine use of nerve stimulator enhances the safety of anesthetic practice. Succinylcholine is the most widely used NMB for endotracheal intubation in Europe (85.8%) and the United States (92.8%), unlike in Korea (2.2%); however, rocuronium is used most frequently for surgical relaxation in all three regions (Europe, 75.8%; United States, 89.6%; Korea, 64.4%). Regarding use of non-depolarizing NMBs, only 18% of European respondents and 34.2% of American respondents answered that they use an anticholinesterase routinely, which is contrast to our result (94.2%).
The one of limitation in this study is relatively low response rate. We sent only one set of e-mails to Korean anesthesiologists without any further reminders or follow-up e-mail or calls. We are willing not to avoiding complaints from respondents. Therefore, we received only 86 completed surveys, resulting in a response rate of 2.3%.
In summary, Korean anesthesiologists prefer rocuronium as an NMB, pyridostigmine as an antagonist, and clinical signs as an assessment tool for recovery from paralysis. The attitude towards the clinical importance of PRNB and the usefulness of neuromuscular monitoring is favorable, with a large percentage of respondents displaying awareness on these issues (87.7% and 76.3%, respectively). However, the distribution of quantitative TOF monitors and their application is relatively low (20.7%). To change this attitude, regular and repeated publicity and education are required, in addition to adequate distribution of neuromuscular monitoring devices and routine clinical use.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download