INTRODUCTION
Many patients suffer from severe anxiety owing to the stress of undergoing surgery and anxiety is highest before the induction of anesthesia [
1]. Sympathetic hyperactivity caused by this type of anxiety is worsened by intubation and surgical stimulations, eventually causing an increased heart rate (HR), vascular resistance, and decreased cardiac output (CO) [
2]. Therefore, one of the key purposes of anesthetic management is to provide appropriate sedation while maintaining hemodynamic stability prior to intubation. Dexmedetomidine (DEX) is an α2-adrenergic receptor agonist that blocks sympathetic outflow and weakens the sympathetic response to intubation and surgery. DEX reduces anxiety and provides sedation by acting on the α
2A and α
2C receptors of the locus coeruleus [
3–
6]. Furthermore, its analgesic effect has been reported to reduce the postoperative opioid requirement by 30% to 50% [
7]. Despite its ideal characteristics as a pre-anesthetic treatment agent, continuous administration of DEX at the loading dose can lead to dose-dependent bradycardia and hypotension, thereby limiting the use of the drug [
5]. Therefore, in this study, we assessed the effects of administering DEX over 10 minutes at a dose of 0.5 μg/kg prior to induction of anesthesia in patients undergoing laparoscopic cholecystectomy to assist in providing sedation for patients prior to surgery. Additionally, we assessed the effects of DEX administration on cardiovascular indices, recovery, and analgesic requirements after surgery.
Go to :

MATERIALS AND METHODS
This study was a prospective, double blind, randomized, placebo controlled clinical study with the approval from Institutional Review Board of Eulji University Hospital (IRB-2016-08-014-001). Sixty patients aged 20 to 60 years who were scheduled to undergo laparoscopic cholecystectomy (American Society of Anesthesiologists grade I or II) were included. Pregnant patients, patients with medical conditions of the heart, liver, renal system, or lungs, and patients with a history of allergies to medication were excluded. Patients were randomly assigned via computer-generated random numbers into either the DEX group (group D, n = 30) or the saline group (group S, n = 30). Premedication was not provided. When the patient arrived at the operating room, electrocardiography was performed and HR, peripheral oxygen saturation (SpO
2), mean arterial pressure (MAP), and bispectral index (BIS) were measured. To observe changes in hemodynamic variables (CO and systemic vascular resistance [SVR]), noninvasive cardiac monitoring (ICON
®, Cardiotronic
®, USA) was performed by applying four electrodes, according to the manufacturer’s manual. For drug administration, 4 μg/ml DEX and 0.9% normal saline were loaded into 50 ml syringes. The appropriate medications were prepared in identical syringes by a nurse who was not involved in the study. All patients and investigators were blinded to the group allocation. After baseline parameters were measured, DEX (0.5 μg/kg) or normal saline was administered over 10 minutes. After completion of the drug infusion, the degree of sedation was assessed using BIS and the Ramsay sedation scale (RSS) as follows: 1 = anxiety and complete awake, 2 = completely awake, 3 = awake but drowsy, 4 = asleep but responsive to verbal command, 5 = asleep but responsive to tactile stimulus, and 6 = asleep and not responsive to any stimulus. In cases where SpO
2 was below 90% and RSS was over 4, 4 L/min O
2 was administered via a mask. Propofol (2.0 mg/kg) and rocuronium (0.5 mg/kg) were used to induce anesthesia and neuromuscular block. Intubation was performed using a cuffed endotracheal tube and anesthesia was maintained by manipulating desflurane to maintain BIS in a range between 40 and 60. During mechanical ventilation, end tidal CO
2 was maintained between 35 and 45 mmHg. Intra-abdominal pressure during the surgery was maintained between 12 and 14 mmHg. The end of surgery was defined as the time point when the surgeon finished complete skin closure. At this point, desflurane was discontinued and pyridostigmine (0.2 mg/kg) and glycopyrrolate (0.2 mg/5 mg of pyridostigmine) were injected. Extubation was performed if the patient exhibited a regular breathing pattern and reacted to verbal commands. HR, MAP, SpO
2, CO, and SVR were recorded at baseline (T0); 5 minutes after DEX or saline infusion (T5); after completion of the DEX or saline infusion (T10); at intubation (T15); at the end of surgery (ES); and at extubation (ET) (
Fig. 1). The time interval between ES and ET was recorded. The modified Aldrete recovery score (MARS) and visual analogue scale (VAS) score for pain were recorded 15 and 30 minutes after entering the recovery room. In cases of bradycardia (HR < 45 beats/min), atropine (0.01 mg/kg) was given. If the patient exhibited a MAP < 50 mmHg, ephedrine (5 mg) was injected. If the patient exhibited severe pain (> 6 VAS) in the recovery room, fentanyl (50 μg) was administered and if the VAS score change within 5 minutes was less than 3, an additional injection of 50 μg of fentanyl was administered. Occurrences of adverse effects, such as nausea, vomiting, bradycardia, hypotension, and dry mouth, were recorded.
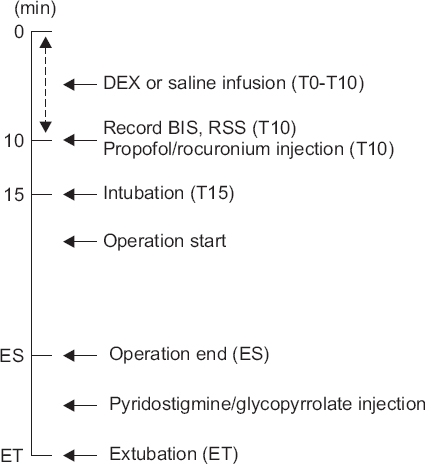 | Fig. 1Anesthesia protocol. T0: baseline, T10: completion of the dexmedetomidine (DEX) or saline infusion, T15: intubation, BIS: bispectral index, RSS: Ramsay sedation scale, ES: end of surgery, ET: extubation time. 
|
Statistical analysis
The primary outcome was the level of sedation as assessed using BIS and RSS after completion of the drug infusion. Secondary outcomes included the comparison between groups of the changes in HR, MAP, CO, and SVR. Statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., USA) and G*Power (version 3.0, Faul, Erdfelder, Lang, and Buchner, 2007) was used to calculate sample size. To detect a 20% reduction from the initial BIS score, which indicates light sedation, before and after DEX infusion, with a power of 80% at the 5% significance level, each group required 27 subjects. After considering a possible drop-out rate of 10%, 30 subjects were included per group. Data are shown as mean ± standard deviation and P < 0.05 was considered significant. The Student’s t-test was used for the between group comparisons of age, weight, height, operation time, and extubation time. To assess sex, a chi-square test was used. The paired t-test was used to evaluate BIS. The Mann-Whitney U test was used to evaluate RSS, MARS, and VAS scores. Repeated measures ANOVA with the Bonferroni correction for multiple comparisons (P < 0.017 was considered significant) was used to assess HR, MAP, CO, and SVR. The Shapiro-Wilk test was used to check the normality of the data. Sphericity was evaluated using the Mauchly test of sphericity.
Go to :

RESULTS
Sixty-two patients were assessed for eligibility, however, two patients dropped out because they changed their minds about sedation during the operation. Therefore, the data were obtained from 60 patients.
There were no significant differences in weight, height, age, sex, or operation time between group D and group S (
Table 1). After completion of the DEX infusion, BIS was significantly lower than baseline values in group D (97.1 ± 2.4, 83.8 ± 4.8; P < 0.001). RSS measurement after completion of DEX or saline was 1 for all patients in group S, whereas 17 patients in group D had an RSS measurement greater than 1 (RSS 1 = 13 patients, RSS 2 = 8 patients, RSS 3 = 8 patients, RSS 4 = 1 patient). There was a significant difference between the two groups (P < 0.001). BIS reduction did not reach 20%, but RSS-rated sedation was appropriate.
Table 1
|
Variable |
Group D (n = 30) |
Group S (n = 30) |
|
Age (yr) |
48.7 ± 11.6 |
44.9 ± 12.2 |
|
Sex (M/F) |
19/11 |
19/11 |
|
Weight (kg) |
70.4 ± 11.8 |
68.7 ± 10.6 |
|
Height (cm) |
166.6 ± 7.3 |
164.7 ± 10.3 |
|
Operation time (min) |
58.0 ± 3.4 |
57.0 ± 3.6 |
|
Extubation time (s) |
220.3 ± 67.3 |
236.3 ± 86.2 |

The change in HR after administration of DEX or saline was different between the groups. At the end of the DEX infusion, HR was lowest and there was a significant difference between the two groups (group D: 61.2 ± 10.7 beats/min, group S: 71.3 ± 12.8 beats/min; P < 0.001;
Fig. 2). This tendency persisted even after the end of the drug infusion. The changes in MAP were different between the groups (P = 0.006), but at the end of the drug infusion, unlike HR, there was no significant difference between the groups (group D: 87.5 ± 15.0 mmHg, group S: 88.7 ± 11.0 mmHg; P = 0.139;
Fig. 3). Similar to HR, MAP was consistently lower in group D than in group S after the drug infusion.
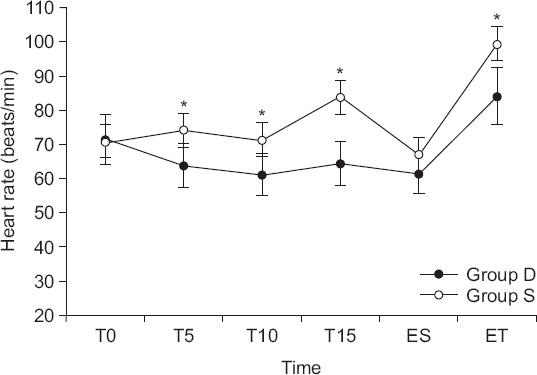 | Fig. 2Changes in heart rate in the two groups. Group D: The group injected with 0.5 μg/kg of dexmedetomidine (DEX). Group S: The group injected with 0.9% normal saline. T0: baseline, T5: 5 minutes after DEX or saline infusion, T10: completion of the DEX or saline infusion, T15: intubation, ES: end of surgery, ET: extubation time. *Significant difference between the two groups (P = 0.017). 
|
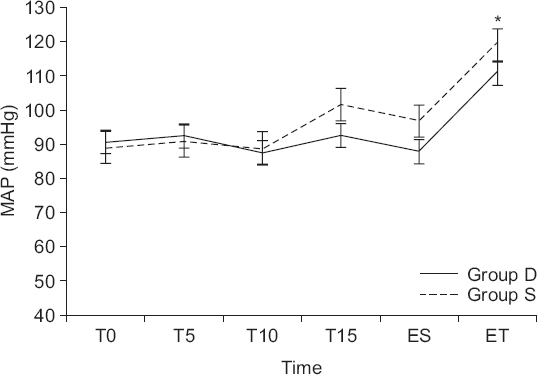 | Fig. 3Changes in mean arterial pressure (MAP) in group D. Group D: The group injected with 0.5 μg/kg of dexmedetomidine (DEX). Group S: The group injected with 0.9% normal saline. T0: baseline, T5: 5 minutes after DEX or saline infusion, T10: completion of the DEX or saline infusion, T15: intubation, ES: end of surgery, ET: extubation time. *Significant difference between the two groups (P < 0.017). 
|
Changes in CO and SVR were significantly different between the groups (CO: P < 0.017, SVR: P < 0.001;
Table 2). CO was lowest at the end of the DEX infusion and was consistently lower than that in group S, whereas SVR was highest at the end of the DEX infusion, but was similar to that in group S after the subsequent measurements.
Table 2
Cardiovascular Parameters
|
Variable |
T0 |
T5 |
T10 |
T15 |
ES |
ET |
|
Group D |
CO |
5.27 ± 1.42 |
4.91 ± 1.43 |
4.36 ± 1.38 |
4.95 ± 1.84 |
4.76 ± 1.73 |
6.18 ± 1.78 |
|
SVR |
1,589.3 ± 466.5 |
1,657.8 ± 482.0 |
1,803.4 ± 568.1 |
1,683.1 ± 511.5 |
1,664.1 ± 567.3 |
1,349.7 ± 391.8 |
|
Group S |
CO |
5.23 ± 1.28 |
5.38 ± 1.09 |
5.23 ± 1.14 |
5.54 ± 1.38 |
4.89 ± 1.07 |
6.82 ± 1.80 |
|
SVR |
1,486.2 ± 396.2 |
1,432.7 ± 362.2 |
1,428.4 ± 365.8 |
1,419.3 ± 497.9 |
1,692.0 ± 532.8 |
1,421.7 ± 474.5 |

There was no significant difference in the MARS value measured in the recovery room at 15 minutes between the two groups, but the value was higher in group S (group D: 7.10 ± 0.75, group S: 7.40 ± 0.77; P = 0.190). At 30 minutes, all subjects from both groups exhibited a MARS value over 9.
VAS scores for pain measured 15 minutes after entering the recovery room were significantly lower in group D than those in group S (P < 0.001). The number of patients who required an injection of fentanyl was higher in group S (21 patients) compared to that in group D (7 patients). In addition, whereas all 7 patients from group D were administered only a 50 μg dose, 7 out of 21 patients from group S received a higher dose of 100 μg. There were 2 patients in group S in whom O2 saturation decreased after fentanyl injection. The lowest value was 89% and the patient recovered with mild stimulation. Only 1 patient from group D suffered from nausea and dizziness. There were no other adverse effects, such as bradycardia, hypotension, or dry mouth.
Go to :

DISCUSSION
Our results suggested that administration of 0.5 μg/kg of DEX over 10 minutes before induction of anesthesia was effective for sedation and minimized changes in hemodynamic variables after intubation. Even so, there was no difference in postoperative recovery between the two groups.
DEX, unlike propofol or benzodiazepines, acts on an endogenous sleep-promoting pathway to exert its sedative effect. The effect mirrors natural sleep and the patient is easily awakened by stimulation [
5]. These characteristics are exhibited within 5 minutes of an intravenous injection of 0.5 to 1.0 μg/kg of DEX [
8]. In our study, we administered a loading dose of 0.5 μg/kg of DEX over 10 minutes before induction of anesthesia, without sustained infusion, and assessed the degree of sedation using BIS and RSS values. Light sedation was indicated by a BIS score between 85 and 80 in the preliminary study, which corresponded to a 20% reduction in baseline values. The desired level of sedation according to RSS was between 2 and 3; at this level, the patient was awake and responsive to verbal commands. In our study, RSS-rated sedation was appropriate, but BIS reduction did not reach 20%. This suggested that when the patient’s level of sedation was assessed using BIS, it would be more appropriate to evaluate the absolute value rather than the reduction of 20% or more from baseline values. Additionally, a level between 85 and 90 is appropriate, as indicated by other studies [
9].
DEX, even at a significantly high concentration, does not cause respiratory depression [
10,
11]. In our study, 2 patients exhibited reduced O
2 saturation during injection (reduced to 95% and 97%); both naturally recovered without additional treatment. BIS values at the time of reduced O
2 saturation were 79 and 75, respectively, and we assumed that excessive sedation, indicated by these low BIS values, resulted in reduced O
2 saturation. Our findings suggested that a one-time injection of 0.5 μg/kg of DEX may result in a temporarily reduced O
2 saturation within 10 minutes of injection, which emphasized the need for continuous monitoring of the patient during administration.
Previous studies reported that use of DEX prior to general anesthesia lowered the required dose of thiopental and propofol by 37% and 80%, respectively [
12,
13]. In our study, the same amount of propofol (2.0 mg/kg) was administered to both groups and group D exhibited a 20% lower BIS value than group S did. Therefore, we believe that if 0.5 μg/kg of DEX is injected before induction of anesthesia, a reduced amount of propofol should be required [
14].
DEX acts on central and peripheral sympathetic nerve endings (terminal) and blocks noradrenaline release, providing a sympathetic nerve blocking effect [
15,
16]. This effect allows for hemodynamic stabilization in situations that are known to cause sympathetic hyperactivity, such as intubation, surgical stimulation, or extubation [
17]. Similarly, in our study, HR after intubation decreased by 10% compared to baseline in group D and blood pressure after intubation increased by 2% compared to baseline values. Therefore, these results indicated that DEX stabilized HR and blood pressure.
Overall, DEX at a dose of 1 μg/kg improved intubating conditions and stabilized hemodynamic changes following anesthetic induction, which was performed using propofol (2 mg/kg) and remifentanil (1.5 μg/kg) without a neuromuscular blocking agent [
18].
Known side effects of DEX include hypotension, hypertension, and bradycardia. Depending on the amount of DEX injected, an up to 27% reduced blood pressure and 17% reduced heart rate and cardiac output were observed [
19]. Hypertension often occurred when a high dose of DEX was injected, resulting from peripheral vasoconstriction owing to peripheral α
2B receptor stimulation. In addition, the occurrence of hypotension and bradycardia was dose-dependent [
20]. Therefore, in this study, we used a dose of 0.5 μg/kg because this was the dose found to have the least effect on hemodynamic variables [
19]. We also injected over a period of 10 minutes to minimize elevations in MAP and decrease the occurrence of hypotension and bradycardia. Although we did not observe an elevation in MAP after drug injection, HR continuously decreased during the injection and was decreased by 15% at the end of the injection. These findings agreed with those from a previous study that indicated that IV injection of DEX at low doses (0.25 and 0.5 μg/kg) over 2 minutes assisted in reducing bradycardia [
12]. Therefore, injecting over a period of 10 minutes may have been key for minimizing side effects.
The cardiovascular variables were measured using an electrical bioimpedance non-invasive CO monitor, ICON
® measured values included CO and SVR. CO values measured at 10 minutes time points were significantly lower in group D than group S. The decrease in CO after completion of the drug injection was dependent on a reduction in HR. Therefore, based on a previous study by Ahn et al. [
21] that suggested that pre-operative treatment with atropine lowered the frequency of bradycardia, we believe pre-operative atropine treatment may effectively reduce CO. SVR increased in group D at T10 and T15, indicating that even when the 0.5 μg/kg dose was injected over 10 min, SVR increased. The increase in SVR was due to the decrease in CO and CVP required for SVR measurement was set to an arbitrary value based on the assumption that the patient’s fluid status did not change. Because SVR values measured at the end of surgery and at the time of extubation in group D were not different from those in group S, this effect presumably disappeared within 1 hour.
Blood pressure was reported to be lowest between 60 and 120 minutes after DEX injection [
22]. In our study, blood pressure was lowest at the end of surgery and a continuous effect on blood pressure was thought to have affected the difference in blood pressure between groups S and D after extubation.
Because the half-life of DEX is 2 hours, we assumed that, after injection, a sedative effect would persist throughout the surgery or recovery. We therefore assessed the effect of sedation on extubation [
23]. There was no delay in extubation owing to a sedative effect in this study. In fact, time until extubation was shorter, although not significantly, in group D (220.3 ± 67.3 s) compared to that in group S (236.3 ± 86.2 s). These results suggested that use of DEX had no effect on the recovery of spontaneous breathing after general anesthesia. However, the amount of inhaled anesthetic used to maintain the same BIS level may have been different in each group, which was a limitation of our study. When using the same concentration of inhaled anesthetics, it may take more time to awaken in group D and additional studies are needed to confirm this. There was no significant difference in MARS values in the recovery room measured at 15 minutes between the two groups, but the value was low in group D, specifically in the “consciousness” category. Similarly, MARS values measured after 30 minutes were low in the “consciousness” category, indicating that the sedative effect of DEX persisted, even until the patient left the recovery room. Therefore, it is necessary to continuously evaluate the patient’s level of consciousness, even after leaving the recovery room.
The analgesic sparing effect from the use of DEX is a well-known phenomenon. This effect is not due to action at the opioid receptor, but rather the stimulation of α
2A and α
2C receptors in the spinal cord; reduced secretion of the pain transmitters, substance P and glutamate; and interneuron hyperpolarization [
24]. Seven patients in group D required a fentanyl injection after surgery, which was significantly less than in group S, in which 21 patients required a fentanyl injection. Furthermore, whereas all 7 patients in group D required only 50 μg of fentanyl, 7 out of 21 patients in group S required a higher dose of 100 μg. These analgesic effects are likely the combined result of a preventive effect, a direct analgesic effect, and persistent sedation in the recovery room. The level of reduction in the required analgesic dosage was similar to that in a previous study, which suggested that a one-time injection of DEX reduced the amount of postoperative analgesics required by 50% [
25].
When the infusion was completed, only one patient from group D suffered from nausea and dizziness. No other side effects were observed.
In conclusion, infusing 0.5 μg/kg of DEX over 10 minutes before induction of anesthesia was effective for sedation in patients undergoing surgery. Furthermore, hemodynamic variables were stable at the time of intubation and postoperative side effects, such as respiratory depression or delayed extubation, were not observed. However, patients should be monitored for potential hypoperfusion caused by reduced CO and increased SVR and preparations should be made for a possible reduction in HR. The amount of opioid required for postoperative pain management was reduced and drug-related side effects were minimal. Patients recovered without further assessment. Therefore, based on these characteristics, DEX could be a useful supplementary sedative when injected before general anesthesia.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download