This article has been
cited by other articles in ScienceCentral.
Abstract
Background:
The authors sought to determine whether a shallow needle approach to the axillary artery would improve complete sensory blocks of median, radial, and ulnar nerves as compared with a perpendicular approach when transarterial axillary block is performed using a scalp vein needle (23G, 3/4’).
Methods:
Fifty-four patients were allocated equally to a perpendicular group (the PA group) or a shallow approach group (SA group). Sensory and motor scores were evaluated and compared in the two groups at 5-minute intervals for 20 minutes after block. The main outcome variables were rates of blockage of median, radial, and ulnar nerves.
Results:
Excellent block rates (defined as completion of surgery using brachial plexus block alone) were obtained in both groups (SA group 77.8% vs. PA group 70.3%, P = 0.755). However, the rate of blockage of all three nerves was significantly higher in the SA group (74% vs. 40.7%, P = 0.013). Furthermore, the rate of complete sensory block of the radial nerve at 20 minutes was significantly greater in the SA group (85.2% vs. 59.3%, P = 0.033).
Conclusions:
A shallow needle approach to the axillary artery resulted in a significantly higher median, radial, and ulnar nerve block rate at 20 minutes after LA injection than a perpendicular approach.
Keywords: Scalp vein needle, Transarterial axillary brachial plexus block
INTRODUCTION
Ultrasound is a popular method of guidance when performing axillary brachial plexus block, but ultrasound equipment is not always available [
1], and thus, it is important that those responsible are able to perform the procedure in the absence of guidance.
Transarterial axillary brachial plexus block (TAAB) is well-established blind technique for achieving safe and reliable anesthesia of the upper extremities [
2,
3]. The technique is based on the anatomical knowledge that infraclavicular parts of the brachial plexus encircle the axillary artery within the tubular fascial sheath [
4]. TAAB is easily performed by even the less experienced, but not uncommonly, incomplete blockade is encountered [
2-
4].
TAAB failure is usually attributed to malpositioning of local anesthetic (LA), and its subsequent diffusion away from the artery beyond the brachial plexus sheath into muscle [
1]. When the TAAB is performed, if the needle tip is positioned immediately beyond the axillary artery, there is a high probability of intra-sheath needle position and a correspondingly low risk of injecting LA outside the brachial plexus sheath [
1].
We hypothesized when a scalp vein needle (23 G, 3/4’) is used for TAAB, that a shallow needle approach to the axillary artery would improve the accuracy of LA delivery within the brachial plexus sheath as compared with a perpendicular approach (Figs.
1A and
1B).
Fig. 1
Perpendicular approach group (PA group) versus the shallow needle approach group (SA group). When the scalp vein needle (23 G, 3/4’) was advanced into the axillary artery, the needle was directed perpendicular to the axillary artery in the PA group (A), whereas in the SA group, a shallow needle approach to the axillary artery was used (B).
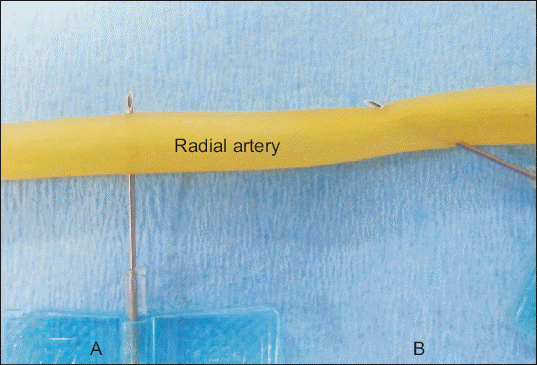
materials and Methods
Fifty-four patients scheduled for surgery of the hand and wrist, were enrolled in the study, after obtaining ethical approval and receiving written informed consent from participants. Patients were aged from 18 to 80 years and were of American Society of Anesthesiologists (ASA) physical status I to III. The exclusion criteria applied were as follows; preexisting neuropathy in the operated limb, ASA > III, coagulation disorders, known allergy to local anesthetics, local infection at the puncture site, multiple injuries, chronic pain history, pregnancy, a body mass index ≥ 35 kg/m2, failure to cooperate, and refusal to participate. Data were collected from January 2015 to June 2015.
Patients were randomized to a perpendicular approach group (PA group, n = 27) or a shallow needle approach group (SA group, n = 27). Randomization was performed using a computer-generated random number table. Patients were not informed of group allocations. Supplemental oxygen (nasal cannulae at 4 L/min) and standard monitoring (noninvasive blood pressure, electrocardiogram, and pulse-oximetry) were applied throughout the procedure; anxiolysis was not established. All nerve blocks were performed by one of two second-grade anesthesiology residents (both had performed more than 20 transarterial axillary blocks, and were supervised by experienced regional anesthesia staff). If, after 10 minutes of attempting a nerve block, a resident could not identify arterial pulsation, more experienced staff took over, and the procedure was not included in this study. To check pulses, residents applied adequate pressure on the axillary artery. The primary end point of this study was the rate of complete sensory block of all three nerves (median, radial, and ulnar nerves) at 20 minutes after LA injection.
All blocks were performed using a blind, transarterial approach, and LA was achieved using 1.5% lidocaine and 1 : 200,000 epinephrine. The technique used was as follows [
1,
5]. The patient’s arm was extended perpendicular to the body and abducted to expose the axilla, and the elbow was then flexed at 90 degrees. The axillary artery was palpated as high in the axilla as possible using gentle pressure [
2], and punctured using a scalp vein needle (23 G, 3/4’, Japanese Medical Supply [K]) until arterial blood was noted in an extension tube open to air. At this point, the scalp vein needle was advanced perpendicularly into the axillary artery until blood flow ceased (PA group) (
Fig. 1A). On the other hand, in the SA group the needle was advanced longitudinally at a shallow angle with respect to the axillary artery in the same manner, until blood flow ceased (
Fig. 1B). At this point, the syringe was connected to an extension tube and 15 ml of LA was injected in 3–4 ml increments after intermittent negative aspiration. After LA injection, the syringe was disconnected, and the needle was withdrawn perpendicularly in PA group, or longitudinally at a shallow angle in SA group until blood flow ceased. At this point, the syringe was reconnected and after negative aspiration another LA 15 ml was injected. Digital pressure was then maintained over the axillary artery for 5 minutes by an independent observer, and 5 ml of LA was directly injected into the coracobrachialis muscle to achieve a musculocutaneous nerve (MCN) block. In addition, a proximal subcutaneous injection of 5 ml of LA was administered along the medial aspect of the distal axilla to provide tourniquet analgesia. Nerve stimulation was not used. The total volume of LA used was 40 ml in both groups.
Brachial plexus block was evaluated every 5 minutes for 20 minutes after LA injection by an independent observer unaware of group allocations. Sensory block was evaluated using an alcohol swab on dermatomes of the ulnar (fifth finger), median (palmar aspect of the second finger), radial (dorsum of the hand between the thumb and second finger), and MCN (lateral aspect of forearm) nerves. Patients quantified sensory block level using the following scale: 2 = normal sensation, 1 = blocked hand less cold than the unblocked hand, 0 = no sensation. Complete sensory block was defined as a score of 0 in ulnar, median, and radial dermatomes. Motor block was assessed by applying the following scale to whole arms: 2 = no paresis, 1 = partial paresis, and 0 = complete paresis. After completing the evaluation, patients were transported to the operating room for surgery.
Block performance time was defined as time from completing the sterile preparation to final withdrawal of the scalp vein needle. Onset time was defined as time required to obtaining complete sensory block of all three nerves. Anesthesia grade was assessed after surgery using a 4-point scale, where: excellent = completion of surgery with only brachial plexus block; good = when intravenous (IV) ≤ 100 μg fentanyl was needed, insufficient = when additional ulnar nerve block was performed at the elbow level, but surgery was completed successfully; and failure = when general anesthesia was required to complete surgery. When a patient requested sedation during surgery, midazolam 2–5 mg was administered after confirming no pain at the incisional site based on anesthesiologist’s decision (anesthesiologists were also unaware of group allocations).
In a preliminary study, complete sensory block of all three nerves was achieved in 4 of 7 in a control group (perpendicular approach), but in 6 of 7 in an experimental group (shallow needle approach) at 20 minutes after LA injection. Power analysis showed 24 patients were required per group for an α value of 0.05 and a power of 90%, and thus, 27 patients were recruited per group to cope with a possible dropout rate of 10%. Results are presented as mean ± standard deviations, medians [interquartile ranges], or as numbers (%). The statistical analysis was conducted using SPSS ver. 12.0 for Windows (SPSS Inc., USA). The Chi-squared test was used to analyze categorical data, and the student’s unpaired t test to analyze continuous data. P values of < 0.05 were considered statistically significant.
RESULTS
Patient demographic data are provided in
Table 1. No significant difference was observed between group demographics. In one patient in each group, arterial pulsation was not detected, and thus, these patients were excluded from the analysis.
Table 1
Patient Characteristics in the Two Study Groups
|
PA group (n = 27) |
SA group (n = 27) |
P value |
|
Age (yr) |
46 ± 15 |
49 ± 15 |
0.531 |
|
Sex (M/F) |
19/8 |
12/15 |
0.054 |
|
Height (cm) |
168 ± 8.6 |
165 ± 8.4 |
0.201 |
|
Weight (kg) |
68 ± 11.7 |
66 ± 9.0 |
0.467 |
|
ASA PS class (I/II/III) |
13/12/2 |
17/10/0 |
0.257 |
Experimental data are presented in
Table 2. Block onset times were not significantly different in the SA and PA groups (10 [6.25–20] vs. 15 [15–20] min, respectively, P = 0.134) at 20 minutes after LA injection. However, rate of blockage of all three nerves at 20 minutes was significantly greater in the SA group (74% vs. 40.7%, P = 0.013). No significant intergroup difference was observed between anesthetic grades at the end of surgery.
Table 2
Intra-operative Group Comparisons
|
PA group (n = 27) |
SA group (n = 27) |
P value |
|
Type of surgery |
|
|
|
|
Hand/wrist |
23/4 |
17/10 |
0.062 |
|
Fracture/non-fracture |
6/21 |
8/19 |
0.535 |
|
Block performer (A/B) |
13/14 |
12/15 |
0.785 |
|
Surgery time (min) |
40 [20–62] |
40 [32–55] |
0.449 |
|
Tourniquet time (min) |
41 [23–60] |
40 [30–50] |
0.381 |
|
Block performance time (min) |
5 [4–6] |
5 [4–6] |
0.477 |
|
Onset time (min) |
15 [15–20] |
10 [6.25–20] |
0.134 |
|
Rate of all 3 nerves blocked (n) |
11/27 (40.7%) |
20/27 (74%) |
0.013 |
|
Anesthesia grade |
|
|
|
|
Excellent/good/ insufficient/fail |
19/6/1/1 |
21/5/0/1 |
0.755 |
|
Sedative/analgesic drugs (total dosage) |
|
|
Midazolam (mg) |
46 |
29 |
0.597 |
|
Fentanyl (μg) |
550 |
500 |
0.860 |
Numbers of patients that achieved a sensory or motor score of 0 at 20 minutes after LA injection are presented in
Table 3. Complete sensory block rates of radial nerves (at 10 and 20 minutes post-injection) and of all three nerves (at 5, 10, and 20 minutes post-injection) were significantly greater in the SA group.
Table 3
Numbers of Patients that Developed a Sensory or Motor Score of 0 at Different Times after Local Anesthetic Injection
|
PA group |
SA group |
P value |
|
M 5 min |
5 (18.5%) |
9 (33.3%) |
0.214 |
|
M 10 min |
8 (29.6%) |
15 (55.6%) |
0.054 |
|
M 15 min |
16 (59.3%) |
16 (59.3%) |
1.000 |
|
M 20 min |
22 (81.5%) |
23 (85.2%) |
0.715 |
|
U 5 min |
10 (37%) |
8 (29.6%) |
0.564 |
|
U 10 min |
14 (51.9%) |
15 (55.6%) |
0.785 |
|
U 15 min |
18 (66.7%) |
17 (63%) |
0.776 |
|
U 20 min |
21 (77.8%) |
22 (81.5%) |
0.735 |
|
R 5 min |
2 (7.4%) |
7 (26%) |
0.068 |
|
R 10 min |
3 (11.1%) |
14 (51.9%) |
0.001 |
|
R 15 min |
11 (40.7%) |
14 (51.9%) |
0.413 |
|
R 20 min |
16 (59.3%) |
23 (85.2%) |
0.033 |
|
All three 5 min |
0 (0%) |
5 (18.5%) |
0.019 |
|
All three 10 min |
1 (3.7%) |
12 (44.4%) |
< 0.001 |
|
All three 15 min |
8 (29.6%) |
12 (44.4%) |
0.260 |
|
All three 20 min |
11 (40.7%) |
20 (74.1%) |
0.013 |
|
MC 5 min |
3 (11.1%) |
2 (7.4%) |
0.639 |
|
MC 10 min |
6 (22.2%) |
10 (37%) |
0.233 |
|
MC 15 min |
13 (48.1%) |
14 (51.9%) |
0.785 |
|
MC 20 min |
18 (66.7%) |
19 (70.4%) |
0.770 |
|
Motor 5 min |
5 (1.8%) |
8 (29.6%) |
0.340 |
|
Motor 10 min |
10 (37%) |
12 (44.4%) |
0.580 |
|
Motor 15 min |
14 (51.9%) |
16 (59.3%) |
0.584 |
|
Motor 20 min |
15 (55.6%) |
17 (63%) |
0.580 |
Paresthesia during TAAB occurred in one patient in the PA group, and in two patients in the SA group. No case of hematoma formation was observed at any injection site. At one-week follow-up visits, no patient complained of persistent paresthesia, no neurologic sequelae were detected, and no patient complained of tourniquet-related pain or any other complication.
DISCUSSION
The major finding of this randomized, controlled study was that the block rates of median, radial, and ulnar nerves around the axillary artery at 20 minutes after LA injection were significantly greater when a shallow needle approach was used during TAAB.
The rates of ‘excellent’ anesthetic grade (when surgery was finished with only brachial plexus block) were similar in the two groups (SA group 77.8% vs. PA group 70.3%, P = 0.755). However, we considered the rate of blockage of all three nerves more meaningful because failure to block one nerve territory can result in failed anesthesia if surgery is conducted in an area innervated by an unblocked nerve [
6].
To ensure LA spread within the brachial plexus sheath, we performed TAAB as high in the axilla as possible because terminal nerves tend to spread far away from the artery even inside the sheath in the distal upper arm [
2,
7]. In addition, we injected LA immediately after arterial blood flow through the needle ceased by letting a tube be opened to air, to ensure LA was injected as close as possible to the axillary artery [
1,
5].
Scalp vein needles are widely used for nerve blocks or irrigation [
8-
10], and incorporate a siliconized tube and wings that allow the operator to stabilize the relatively short beveled needle [
8]. However, during TAAB, the axillary artery must be palpated for arterial pulsation when the needle is advanced into or withdrawn from the artery, which may cause the needle tip to move beyond the brachial plexus sheath.
TAAB is based on the known anatomical proximities of all three nerves to the axillary artery within the brachial plexus sheath [
7]. Therefore, we hypothesized that a shallow needle approach to the axillary artery (
Fig. 1B) would decrease the distance between the needle tip and the arterial wall, and that it would increase the rate of needle tip being located within the brachial plexus sheath.
Most cases of incomplete anesthesia after TAAB are caused by unreliable blockade of the MCN and radial nerves [
2,
4]. In the present study, the radial nerve was more completely blocked at 10 and 20 minutes after LA injection in the SA group than in the PA group. However, one failure was observed in the SA group, in a patient with a deep laceration of the dorsal wrist. In this patient, median and ulnar nerves were partially blocked.
The present study has a number of limitations that require consideration. First, the direction of needle tip was determined by arm extension and not by axillary artery location, and thus, we were not able to measure angles of needle approach to the axillary artery. We believe that the angle between needle tip and axillary artery in the SA group was around 45 to 60 degrees as shown in
Fig. 1, but it undoubtedly varied due to individual factors. Ultrasound should be utilized in further studies to ensure precise needle advancement and to monitor the procedure.
Second, we blocked the MCN outside the sheath separately [
11-
13], and thus, the MCN was not included in our analysis of complete sensory block rates. However, it was included in the assessment of anesthetic grade, because grading was performed after surgery. Nevertheless, we believe MCN block did not affect our assessment of the effectiveness of TAAB, because MCN sensory block scores were similar in the two study groups (
Table 3). In addition, we did not include surgeries of the anterior-lateral forearm, had we done so complete blockade of the MCN would have confounded results regardless of the success of TAAB [
1].
Third, two anesthesiologists performed all blocks in the present study, and they were not blinded to group allocations. However, the sensory and motor test evaluations were performed by an independent blinded observer. Therefore, we believe unintentional bias had little impact on overall results [
10].
Fourth, we used complete sensory block instead of surgical block, because we evaluated block scores for 20 minutes, which was compatible with ranges of onset times in the two groups [
14]. We chose 20 minutes post-injection for evaluation purposes for reasons of clinical efficacy, and because lack of block development within this time indicates a high risk of inadequate block [
12,
14].
Fifth, the total volume injected to block all three nerves was 30 ml. TAAB is a volumetric technique, and thus, an increase in volume can enhance block quality, although considerations of LA toxicity restrict injection volume [
2,
12]. We believe that more accurate block at tolerable LA doses is possible if anatomical and technical factors are adequately considered, even when a blind technique is used. Furthermore, it should be noted that not even ultrasound-guided block can guarantee complete, safe block [
15].
Sixth, we believed LA would be better distributed in the SA group within the sheath of axillary brachial plexus, but we did not confirm intra-sheath placement of the needle tip or LA distribution by ultrasound or contrast radiography [
1]. Furthermore, we did not consider septae dividing the brachial plexus within the sheath. However, the presence of septae has not been confirmed, and it has been known LA permeates septae and distributes within sheaths [
13,
16,
17].
In conclusion, LA injection using a shallow needle approach to the axillary artery during TAAB was found to have a significantly higher rate of blocking median, radial, and ulnar nerves at 20 minutes post-injection than a perpendicular needle approach. In addition, the shallow needle approach also resulted in a significantly higher rate of complete sensory block of the radial nerve than the perpendicular approach.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download