INTRODUCTION
An endotracheal tube (ETT) cuff plays an important role in preventing aspiration and air leakage during positive-pressure ventilation in the operating room or the intensive care unit and even during tracheal extubation [1]. A high intracuff ETT pressure may effectively seal the ETT cuff. However, a high intracuff pressure can damage the tracheal mucosa due to reduced perfusion pressure, and can result in post-extubation airway symptoms including sore throat, hoarseness, and dysphagia [2-4]. Therefore, an intracuff pressure of 20–30 cmH2O and adjustment of intracuff pressure guided by objective measurement is normally recommended in order to prevent cuff-related complications [5,6].
The intracuff pressure is affected by many factors, such as head and neck positioning, material and type of the ETT cuff used, and the concomitant anesthetic gas [7-12]. Among these, nitrous oxide (N2O) diffuses into the ETT cuff, increasing the intracuff pressure and the risk of tracheal trauma [7-9]. An in vitro study reported differences in the intracuff pressure among five types of ETT cuffs during exposure to 66% N2O [9]. It showed that the time during which the intracuff pressure increased from 20 to 30 cmH2O varied depending on the cuff shape and material used. According to the manufacturer, the cylindrical cuff (Mallinckrodt™ Hi-Lo tracheal tube, Covidien, Ireland) and tapered cuff (Mallinckrodt™ TaperGuard tracheal tube, Covidien) are made of the same material, and have the same thickness. However, the intracuff pressure of the tapered cuff increased less than that of the cylindrical cuff during N2O exposure in simulation conditions [13]. Furthermore, little is known clinically about the effect of a different ETT cuff shape on the intracuff pressure. If the intracuff pressure of the tapered cuff increased less than that of the cylindrical cuff during N2O exposure, the cuff shape would be a cause of the difference in intracuff pressure, and an ETT with a tapered cuff would be a good choice for tracheal intubation during general anesthesia using N2O.
This study aimed to evaluate whether the change in the intracuff pressure of the tapered cuff was different from the cylindrical cuff in patients undergoing general anesthesia using 60% N2O.
MATERIALS AND METHODS
Patients
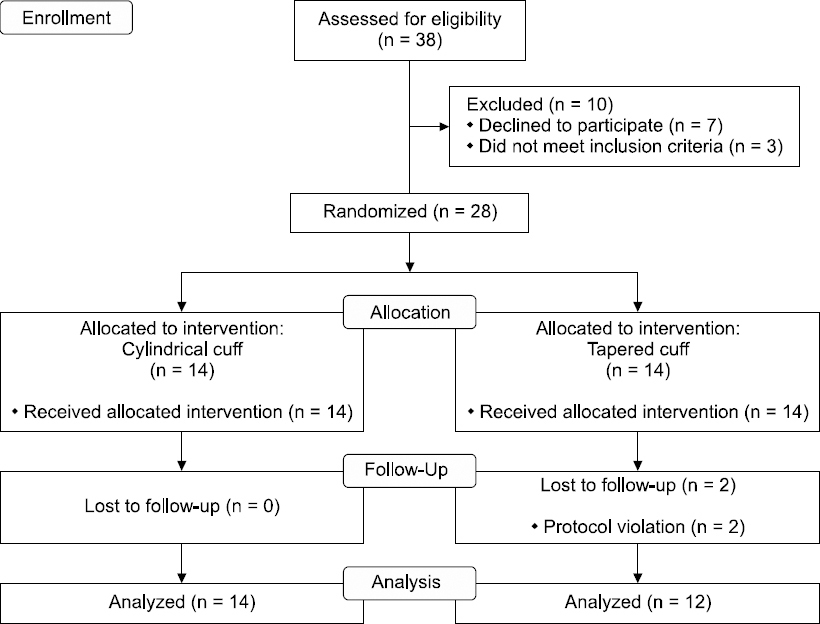
This prospective, randomized, single-blind, parallel-group, clinical study was conducted between August 2014 and February 2015 and followed the CONSORT guidelines for clinical trials (Fig. 1). The study protocol was approved by the Institutional Review Board (Protocol No.: 2013-23), and was registered at ClinicalTrials.gov (Identifier: NCT01812915).
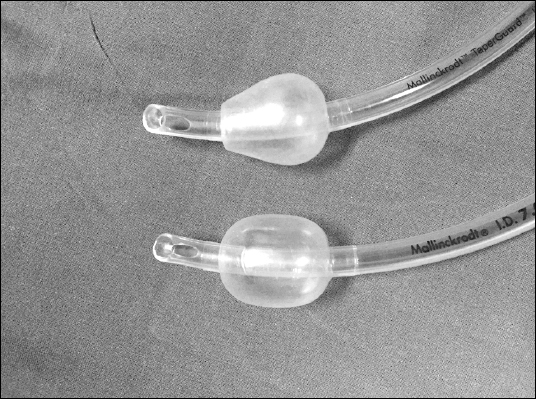
After obtaining written informed consents, we enrolled 28 patients aged 18 to 70 years with American Society of Anesthesiologists physical status I, II, or III and body mass index < 30 kg/m2, who were undergoing elective hand or forearm surgery under general anesthesia in supine with neutral head position. Exclusion criteria were presence of contraindications for N2O, airway-related or pulmonary diseases, difficult intubation, Cormack-Lehane grade > 3, and an anesthetic time of < 1 hour or > 3 hours. The patients were randomly divided into two groups using sequentially numbered, opaque sealed envelopes containing the ETT details. The attending anesthesiologist was asked to pick up the envelope just before the anesthetic induction. Another researcher who was blinded to the group assignment was responsible for recording the intracuff pressure values. For each patient in Group C (n = 14), an ETT with a conventional cylindrical cuff was used; for those in Group T (n = 12), an ETT with a tapered cuff was used (Fig. 2).
Anesthetic management
None of the patients received any premedication. Upon arrival in the operating room, the patients were monitored using electrocardiography, noninvasive blood pressure measurement, pulse oximetry, esophageal temperature probe, and capnography. After pre-oxygenation, general anesthesia was induced by administrating 2 mg/kg propofol and 1 μg/kg remifentanil, and muscle relaxation was achieved with 0.1 mg/kg vecuronium. Female patients were intubated using an ETT with 7.0 mm internal diameter, and male patients were intubated with a 7.5 mm internal diameter ETT. The ETT was secured at the right corner of the mouth. Anesthesia was maintained with sevoflurane at 2–3 vol% and a total fresh gas flow of 2 L/min (60% N2O and 40% oxygen). In previous studies [14-16], the N2O concentration varied between 50% and 70%; hence the middle value (60%) was chosen for the present study. The patients were ventilated with a tidal volume of 6–8 ml/kg and a respiratory rate of 10–12 breaths/min to maintain an end-tidal CO2 of 30–34 mmHg, and a peak inspiratory pressure of less than 18 cmH2O during general anesthesia.
Outcome assessment
The pilot balloon of the ETT was connected to a manometer (VBM cuff pressure gauge, VBM Medizintechnik GmbH, Germany) using a 10 ml syringe via a 3-way stopcock. A syringe was used to inflate the ETT cuff with air until the intracuff pressure reached 20 cmH2O as the baseline. Measurements of the intracuff pressure were performed just before the start of 60% N2O and every 10 minutes afterward until 60 minutes had elapsed. The intracuff pressure was recorded at the end of exhalation, the time at which the intracuff pressure was at its lowest during a ventilatory circle. After 60 minutes, the intracuff pressure was maintained at approximately 20 cmH2O until the end of anesthesia. N2O concentrations, obtained from the anesthesia gas module of GE Datex-Ohmeda Avance S/5™ workstation, were recorded at the end of exhalation every 10 minutes. The incidences of sore throat, hoarseness, and dysphagia were assessed at 1, 6, and 24 hours postoperatively by one researcher who was not aware of the patient’s group allocations. The number of patients using patient-controlled analgesia and the dose of bolus fentanyl administered just before the end of surgery were recorded.
Statistical analysis
The primary endpoint was the intracuff pressure after 60 minutes of 60% N2O exposure. The secondary endpoints were the intracuff pressure at 10, 20, 30, 40, and 50 minutes after N2O exposure, and the incidence of postoperative sore throat, hoarseness, and dysphagia.
The sample size of 12 patients per group was estimated to detect an intracuff pressure difference of 10 cmH2O between Groups C and T, with an assumed standard deviation within a group of 7 cmH2O [17]. The type 1 error was set at 0.05, and the power at 0.9. Twenty-eight patients were allocated to account for dropout. The sample size was calculated using G*Power 3 (Heinrich-Heine University, Germany) [18].
As per the results of the Shapiro-Wilk test, age, height, end-tidal N2O concentration at each time point, and the intracuff pressure after 60 minutes of N2O exposure were compared using an independent t-test. Weight, body temperature, anesthetic time, and the dose of fentanyl were compared using the Mann-Whitney U test. Differences in the number of males and females allocated to the two groups were compared using the Chi-square test, whereas the incidences of sore throat, hoarseness, and dysphagia were analyzed using Fisher’s exact test. Statistical analysis was performed using SAS (Version 9.3, SAS Inc., USA).
RESULTS
In the two groups, the distribution of sex, height, weight, body temperature, anesthetic time, patient-controlled analgesia, and the dose of bolus fentanyl was similar, whereas age showed a difference in distribution (Table 1).
Table 1
Patient Characteristics
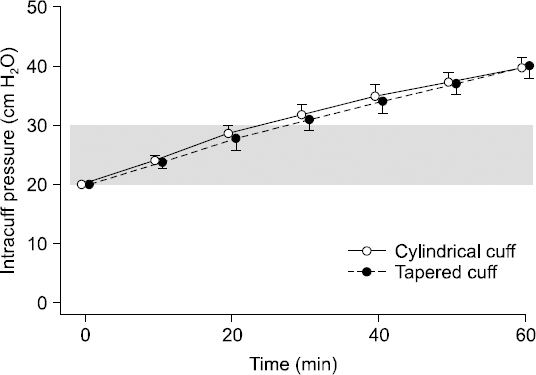
The intracuff pressure at 60 minutes was 40 cmH2O in Group C (95% CI, 36–44) and 40 cmH2O in Group T (95% CI, 35–45, P = 0.895) (Fig. 3); the difference between the two groups was 0 cmH2O (95% CI, −6–5). The lower limit of 95% CI for the intracuff pressures exceeded 30 cmH2O at 60 minutes in both groups.
Fig. 3
Changes in the intracuff pressures of the endotracheal tubes during general anesthesia using 60% nitrous oxide. There is no significant difference between the cylindrical cuff (MallinckrodtTM Hi-Lo tracheal tube, Covidien, Ireland) and the tapered cuff (MallinckrodtTM TaperGuard tracheal tube, Covidien) after 60 minutes of nitrous oxide exposure during general anesthesia (P = 0.895). The dots represent the mean and the vertical bars indicate the standard error of the mean at each time point. The gray band shows the clinically acceptable intracuff pressure.
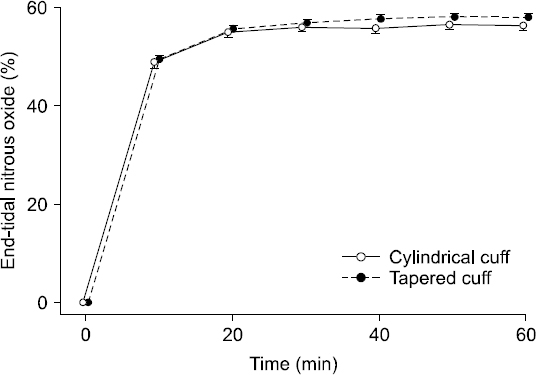
There were no significant differences in the incidence of postoperative sore throat at 1 (Group C vs. Group T, 2 vs. 1; P = 1.000), 6 (2 vs. 0, P = 0.483), and 24 hours (3 vs. 0; P = 0.225). No patients reported hoarseness or dysphagia. The N2O concentrations in Group C were not different from that in Group T at each time point (Fig. 4).
Fig. 4
Changes of nitrous oxide concentrations during general anesthesia. There is no significant difference of nitrous oxide concentration between the cylindrical cuff (MallinckrodtTM Hi-Lo tracheal tube, Covidien, Ireland) and the tapered cuff (MallinckrodtTM TaperGuard tracheal tube, Covidien) for 60 minutes. The dots represent the mean and the vertical bars indicate the standard error of the mean at each time point.
DISCUSSION
This study showed that the intracuff pressure of the ETT did not differ between the cylindrical cuff and the tapered cuffs after 60 minutes of N2O exposure during general anesthesia (P = 0.895). Furthermore, this result was confirmed by the confidence limit (6 cmH2O), which was less than the difference we had expected (10 cmH2O). The intracuff pressure was, therefore, not affected by the cuff shape. Considering the 95% lower confidence limit in intracuff pressure (36 cmH2O in Group C and 35 cmH2O in Group T) at 60 minutes, it is reasonable to assume that the intracuff pressure increased more than 30 cmH2O (the upper limit of clinically acceptable intracuff pressure) before 60 minutes had elapsed. Therefore, continuous or frequent monitoring is recommended even if the duration of N2O exposure is less than 1 hour.
The rate of N2O diffusion into the cuff is affected by many factors, including the partial pressure gradient of N2O inside and outside the cuff, the area that is exposed to N2O, the diffusion time, and the cuff thickness [19]. According to the manufacturer, the cylindrical and tapered cuffs are manufactured using the same material, and has the same thickness; thus, the surface area available for N2O diffusion plays an important role in the increase in intracuff pressure. The surface area may differ depending on the degree of the longitudinal folds in the cuff membrane.
The intracuff pressure in this study seems to be lower than that of a previously published simulation study [13]. The human trachea may not be as rigid as the model trachea; thus, we predict that the human tracheal wall relieves some of the increase in the intracuff pressure. In addition, mucosal secretions may coat the cuff and seal the cuff folds, resulting in a decrease in the area available for N2O diffusion into the cylindrical cuff. This may be the one reason why the intracuff pressure did not differ between cuffs, although the cylindrical cuff was more folded when inflated.
This study does have some limitations. First, we specified the primary outcome analysis prior to initiating the study, on the basis of the independent two sample t-test of the intracuff pressure at 60 minutes of N2O exposure. However, considering that the data demonstrated a linear increase in the intracuff pressures in a time-dependent manner, alternative methods of statistical analysis may be available to further test these data. Second, considering an anesthetic circuit volume and the patient’s functional residual volume, higher flow rates could have raised the concentration of N2O in the lungs more rapidly, hence intracuff pressure changes would have occurred more rapidly. It might have affected the differences in the intracuff pressure resulting from the cuff shape. However, the N2O concentrations in both the groups were not different at each time point (Fig. 4), and the primary endpoint was the intracuff pressure at 60 minutes after the initiation of 60% N2O; hence, we assumed that the effects of the flow rate on the intracuff pressure was insignificant. Lastly, although there were no significant differences in the incidence of postoperative sore throat, it may not be clinically meaningful because the sample size was too small to evaluate the incidence of sore throat properly.
In summary, the intracuff pressure of the TaperGuard™ ETT was not different from that of the Hi-Lo™ ETT after 60 minutes of 60% N2O exposure during general anesthesia. The intracuff pressure can exceed 30 cmH2O within an hour; hence, continuous or frequent monitoring is recommended regardless of the duration of 60% N2O exposure. Additionally, if a lower or higher concentration of N2O is used, the increase in intracuff pressure may be slower or faster, respectively, compared to 60% N2O.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download