INTRODUCTION
Paraganglioma is a rare, highly vascular neoplasm that arises from the neural crest chromaffin cells and closely related to pheochromocytoma. Its prevalence is approximately 0.0002–0.001% in the general population [1]. The tumor originates from the carotid body, Organ of Zuckerkandl and other cells in the autonomic nervous system [2]. Clinical presentation of paraganglioma varies from asymptomatic to catecholamine excess symptoms, such as headache, palpitation, and dyspnea. The variety of clinical presentations makes the diagnosis of this tumor difficult. As a result, most of the extra-adrenal paragangliomas are rarely diagnosed preoperatively [3], and are instead found incidentally during surgical procedures. Catecholamine excess causes life-threatening conditions such as uncontrolled hypertension, tachyarrhythmia, cardiomyopathy, myocardial infarction, and pulmonary edema [4]. Excessive secretion of catecholamines during the surgery can make anesthetic management difficult, and lead to hemodynamic lability. Thus, there is a need for adequate and appropriate management. We reports a case of anesthetic management in a patient diagnosed with retroperitoneal paraganglioma.
Go to : 
CASE REPORT
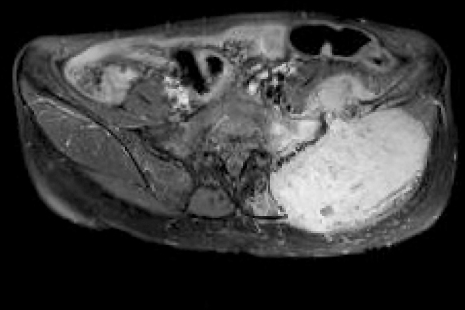
A 55-year-old woman visited the hospital due to the presence of a palpable mass in the left buttock area. The patient was on medication for hypertension, and her blood pressure (BP) was well-controlled. The height of the patient was 162.5 cm, and weight was 49.3 kg. Twenty years ago, she had previously undergone a surgery for pheochromocytoma of the pancreatic tail; no remnant mass was found using abdominopelvic computed tomography (CT). Through magnetic resonance imaging (MRI), a well demarcated mass, 12 cm in sized, was found in the left ilium, with intra- and extra- pelvic soft tissue formation (Fig. 1).
For further evaluation, we requested a radiologist to conduct and ultrasound-guided biopsy and perform a CT scan. From the biopsy results, the mass was diagnosed as paraganglioma. The CT scans revealed an 11-cm large bone mass, invading the left iliac bone and the adjacent muscle. Thus, we planned to perform hemipelvectomy. Additionally, because most paragangliomas are highly vascular neoplasms, embolization was arranged before the surgery to reduce bleeding risk.
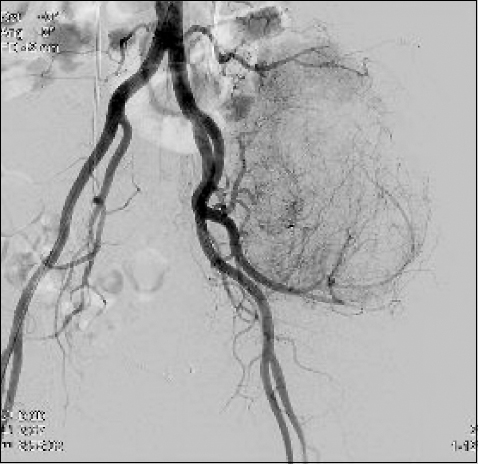
Left internal iliac angiography showed multiple staining branches (Fig. 2). Two minutes after embolization, the patient complained of headache, palpitation, and dyspnea. Her vital signs were as follows: BP, 230/130 mmHg; heart rate (HR), 140 beats/min; and respiratory rate (RR), 40 breaths/min. To treat malignant hypertension, we immediately administered 6 mg of nicardipine, and 100 μg of fentanyl, intravenously. However, neither drugs showed any effect; thus, all the procedures were stopped. With a continuous infusion of nicardipine in the ward, the patient did not show any malignant hypertension symptoms, and eventually became stable.
We thought that an excessive catecholamine production from the tumor is the cause of patient’s symptoms, in order to ascertain this, we performed several tests. The hormonal test, 24-h urine samples, revealed normetanephrine excretion at 30547 μg/day (normal < 444 μg/day), metanephrine excretion at 6340 μg/day (normal < 341 μg/day), and urine vanillymandelic acid at 76.5 mg/day (normal < 7 mg/day). I-123 MIBG scan demonstrated an increased uptake in the left buttock, and muscle invasion of the left iliac bone. A gene study was negative for multiple endocrine neoplasia type 2 (MEN2), von-Hippel-Lindau (VHL), succinate dehydrogenase subunit D (SDHD), and RET genes. Doxazocin and propranolol premedication was planned for 2 weeks before the surgery, and the surgery was postponed.
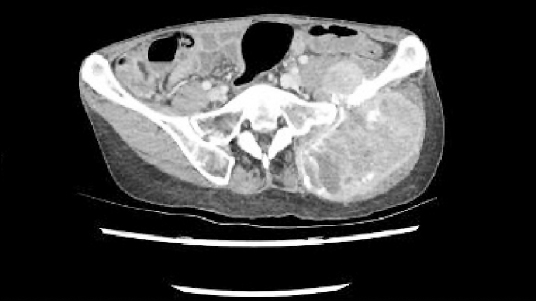
Five months later, the patient revisited our center; an CT scan showed and increase in the size of the mass (Fig. 3). Preoperative embolization and the surgery were scheduled. Preoperative laboratory test results showed no abnormal findings. Two weeks before the planned surgery, we initiated the premedication. Target systolic BP was 90–120 mmHg; doxazosin was started at a dose of 2 mg/day, as oral medication. This was increased to 4 mg/day, according to the required target BP. Propranolol intake was maintained at 20 mg/day, as oral medication, for 3 days before the surgery.
Despite premedication for 2 weeks, the patient suffered from headache and palpitation during embolization. Following this, the patient was moved to the operating room; the patient’s noninvasive BP was 218/114 mmHg and HR was 124 beats/min. Sinus tachycardia on Electrocardiogram and pulse oximetry showed 99% of blood oxygen saturation level. Prior to anesthetic induction, the patient was stabilized by using 1 mg of nicardipine and 50 mg of esmolol. General anesthesia was induced with 150 mg of propofol, followed by a dose of 50 mg of rocuronium, for muscle relaxation. Remifentanil was administered by using a target-controlled infusion system. Target effect-site concentration was 3 ng/ml.
A twenty-gauge angiocatheter was placed at the right radial artery for invasive blood pressure monitoring, and stroke volume variation monitoring. Ultrasound-guided right internal jugular cannulation was performed for monitoring central venous pressure (CVP), and for better fluid management. CVP was around 12–13 mmHg after cannulation.
During the surgery, anesthesia was maintained with 2–3 vol% of sevoflurane in air (1.5 L/min) and oxygen (0.5 L/min). The patient was hemodynamically stable during induction, intubation, and skin incision. Whenever the surgeon touched the mass, the patient showed malignant hypertension and tachyarrhythmia. Hence, we started to administer sodium nitroprusside at 0.5–1.0 μg/kg/min. In cases where the systolic BP was larger than 200 mmHg, we used a bolus of labetalol, 2 mg, or nicardipine, 1 mg. BP and the HR stabilized; thus, these drugs were found to be effective.
After resection of the tumor in the invaded bone, the patient’s hemodynamic parameters suddenly became unstable. Because of massive bleeding and a sudden decrease of catecholamine secretion, the patient exhibited BP as low as 80–90/40–50 mmHg and a slightly increased HR of 90–100 beats/min. CVP also dropped around 5–6 mmHg. Thus, we stopped nitroprusside and remifentanil infusion, and instead started phenylephrine infusion to improve the hemodynamic status of the patient. Additionally, we transfused 8 packs of packed red blood cells, 5 packs of fresh frozen plasmas and 6 packs of platelet concentrates. To support bleeding control, we used tranexamic acid, and 100 ml of 20% albumin for volume expansion. Such procedures were not enough to control the patient’s vital signs. Therefore, so we added 0.03–0.06 μg/kg/min of norepinephrine to stabilize the patient. The bispectral index value was maintained between 30–50, during the surgery.
After the completion of all of the procedures, BP was 100/76 mmHg and HR was 78 beats/min. The patient was moved to an intensive care unit for close monitoring in the intubated state. Total anesthesia time was 8 h; surgery time, was 7 h; Estimated blood loss, 5,000 ml; urine output, 2,360 ml. Infused fluids consisted of 6,850 ml of crystalloids and 100 ml of colloids. Twenty packs of packed red cells, 13 packs of fresh frozen plasma and 18 packs of platelet concentrates were transfused during surgery.
The patient was extubated a day after surgery, and showed no specific hemodynamic abnormality throughout her hospital stay. One month after surgery, several work ups were conducted to find any signs of recurrence or metastasis. Abdominopelvic CT showed a total removal of the mass. In the hormonal test, all catecholamine metabolites were within the normal range.
Go to : 
DISCUSSION
Paraganglioma is a tumor derived from the sympathetic and parasympathetic nervous systems; and therefore, it appears anywhere from the cranial base to the epididymis [4]. Sympathetic paraganglioma is usually functioning and often arises in abdomen, whereas parasympathetic one is usually non-functioning and often arises in the head and neck.
Approximately 30% of all paragangliomas are hereditary. Paraganglioma is associated with genetic syndromes such as neurofibromatosis 1, VHL disease, MEN 1, MEN 2 and SDHD B mutations [5]. Thus, genetic tests are recommended to paraganglioma patients.
A total of 15–35% of paragangliomas are malignant [2]. There are several tools to diagnose malignancy; however, no clear diagnostic criteria exist. A large size, heterogenous density, irregular margins, and distance metastasis, are the signs of malignancy. Tumor marker chromogranin A correlates with size and malignancy; therefore, it is useful for detecting SDHB related paraganglioma [6].
The main manifestation of paraganglioma is hypertension; but it has no specific symptoms. Thus, it is hard to diagnose. Paraganglioma diagnosed incidentally using imaging studies or by mass-effect related symptoms in most patients [3,4]. Conventional imaging modalities, particularly CT and MRI are useful in diagnosis. CT sensitivity is approximately 90% for abdominal paraganglioma. 123I-MIBG scintigraphy can be also helpful. It is usually recommended to obtain two different imaging modalities, an ‘anatomic’ imaging modality (CT-scan or MRI) to assess structural details (tumor size, vascular invasion of renal veins or inferior vena cava, invasion or displacement of adjacent organs), and functional imaging to exclude multiple tumors and the presence of metastases [4].
Twenty four-hours measurement of total or fractionated urine catecholamines and metabolites are useful laboratory evaluation for paraganglioma. Norepinephrine and epinephrine have very short half-lives. Normetanephrine and metanephrine are inactive catecholamine metabolites; thus, they have much more prolonged half-lives. Sampling of 24-h urine metanephrine has a sensitivity of 89.9% and specificity of 99% [7].
Resection of the tumor is the best treatment in symptomatic paraganglioma; however, the surgical procedure itself can be life threatening. Over the last 50 years, there has been a marked decline in perioperative mortality from 40–60% to 0–6% [8].
Functional paraganglioma can be a challenge to anesthesiologists because of its ability to secrete catecholamine. Usually, catecholamine secretion from the paraganglioma is triggered by a direct pressure on the tumor, manipulation during surgery, or touching the mass through the abdominal wall. Catecholamine storm can cause serious complications such as severe hypertension, myocardial infarction, coronary spasm, arrhythmias, cardiomyopathy, stroke and pulmonary edema [4].
Nicola et al. [9] reported that preoperative embolization could reduce the circulating levels of catecholamines and the risk of hemodynamic fluctuations. Preoperative embolization is often performed to reduce bleeding risk. There was a concern that the embolization effect could be reduced, to the level that the collateral feeding vessel might come back by angiogenesis when the surgery was delayed after the embolization.
If malignant hypertension occurs during procedures, the practitioner must decide whether to proceed, or to stop. In our case, at first embolization, we stopped the procedure because the drugs had no effects, and no premedication was administered. At the second event, malignant hypertension occurred. However, we continued because appropriate preoperative pharmacological treatment was administered to restore normal circulatory volume and to prevent release of catecholamines. Deep level anesthesia should be provided to prevent sympathetic activity. Sodium nitroprusside is effective in arteriolar dilatation and in suppressing the hypertensive response due to circulating catecholamines. There have been reports that nicardipine suppresses the secretion of norepinephrine in the paraganglioma [10]. Jeon et al. [11] reported a successful management with labetalol and esmolol in malignant hypertension caused by metastatic adrenal tumor. When wide and precipitation swing occurs in the BP and HR, more short-acting drugs are preferred and recommended than long-acting drugs.
Hypotension can be followed after tumor removal. Abrupt fall in catecholamine levels, which leads to a sudden dilatation of the vasculature, massive bleeding and residual effect of anti-hypertensive drugs are possible causes. Thus, adequate fluid replacement and blood transfusion is needed.
Appropriate cardiovascular premedication is essential for minimizing complications, like arrhythmias, cardiac ischemia and malignant hypertension [12]. All patients with elevated catecholamine levels are prepared for surgery with α-blockers and β-blockers. A salt rich diet is recommended for volume repletion.
Paraganglioma is a rare disease not familiar to most anesthesiologists. In case of functional paraganglioma, it can secrete catecholamines, and causes catecholamine associated problems such as malignant hypertension, tachyarrhythmia and other cardiac complications. An adequate management during the procedure and a proper premedication is an essential for a better prognosis. Thus, a multidisciplinary approach of anesthesiologists with endocrinologists, radiologists and surgeons is needed for better care.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download