INTRODUCTION
Although spinal epidural hematoma is rare, this disease can lead to neurological symptoms or paralysis due to pressure on the spinal cord or cauda equina. It usually occurs after external injuries such as catheter insertion for continuous epidural anesthesia or invasive spine surgeries such as spinal puncture [1]. Epidural hematoma primarily occurs in patients treated with anticoagulants before implementation of epidural blocks or in those with coagulopathy. It rarely occurs in patients without any risk factors. Acute spinal epidural hematomas associated with neurological symptoms are usually treated by surgical drainage. Herein, we report a case of acute thoracic epidural hematoma associated with neurologic symptoms after epidural block in a healthy male without risk factors. We performed drainage of the epidural hematoma using 18-gauge Tuohy needle without surgical intervention. The patient’s neurological symptoms and pain were relieved. He was discharged without sequelae. We also performed literary review for spinal epidural hematoma.
CASE REPORT
A 73-year-old male patient who visited with intermittent intolerable sharp and lancinating pain over the left anterior chest wall and the back for one month. He received 300 mg/day of gabapentin and 1,500 mg/day of valacyclovir orally for 7 days following diagnosis with acute herpes zoster at a local private clinic. The patient did not have any unusual history, including family history and surgical history. He had no systemic diseases such as hypertension, diabetes, and so on. His height and weight were 172 cm and 59 kg, respectively. Results of his blood tests such as activated partial thromboplastin time, prothrombin time, and platelet count all showed normal ranges.
Physical examination revealed allodynia and hyperalgesia over the left T4 and T5 dermatomes. He complained severe pain in the affected area with a score of 8 or 9 on visual analog scale (VAS) where 0 indicated “no pain” and 10 indicated “the strongest pain imaginable.” In particular, he was unable to sleep at night due to severe pain which interfered with his daily life.
Under the diagnosis of post-herpetic neuralgia, the patient was hospitalized and received a patient controlled epidural analgesia for 48 hours via an epidural catheter at T4-5 level. Two days later, the patient was discharged from the hospital for personal reasons. He was given treatment through an outpatient clinic. For the drug therapy, 75 mg of Pregabalin and 37.5 mg of immediate-release tramadol HCl/325 mg acetaminophen were prescribed to be divided into two doses per day, with 10 mg of amitriptyline being added to the regimen to be taken before going to bed. Simultaneously with the drug therapy, fluoroscopy was performed for guidance of an epidural block using the ‘loss of resistance’ method. For the epidural block, 8 ml of 0.8% emcaine was administered in the epidural space around T4-5 spine twice weekly for seven weeks. His hyperalgesia was reduced from 8 points to 3 points on VAS.
The epidural block was performed using 22-gauge needle without any specific problem during the 14th episode. The 15th block was also performed without any specific finding such as dural puncture or bleeding.
The patient was thus sent home one hour after the treatment as usual. His vital signs measured before the procedure were: blood pressure (BP), 131/62 mmHg; heart rate (HR), 68 times per minute; temperature, 36.7°C. His vital signs before being released to return home were: BP, 140/80 mmHg; HR, 70 times per minute; body temperature, 36.8°C. Hypoesthesia or hyperalgesia of the upper or lower limb was not observed.
The patient came back to the emergency room with sudden dorsal pain and dysuria at six hours after returning home. The dorsal pain got worse, exhibiting a VAS of 9 points. Bladder retention was confirmed. Therefore, a urinary catheter was inserted to empty a 1,400 cc volume of urine. Based on neurological examination, the motor grade of lower limbs was 4 points/4 points. He was unable to walk without help. His sensory was intact.
Under the suspicion of acute epidural hematoma caused by epidural block, an urgent thoracic magnetic resonance imaging (MRI) was performed. Acute epidural hematoma over the T3-T6 region was found to press the cord of the T4-5-6 position (Figs. 1 and 2).
Fig. 1
Sagittal T2-weighted image. Acute epidural hematoma (white arrow) at posterior epidural space of T3 to T6 level with severe cord compression at T4-5-6 level.
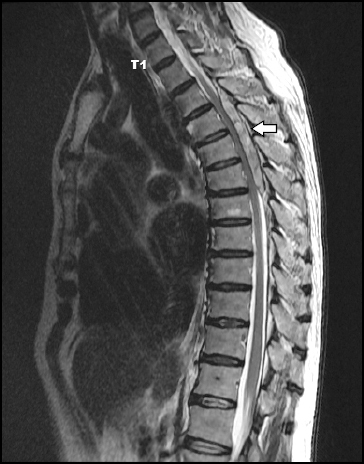
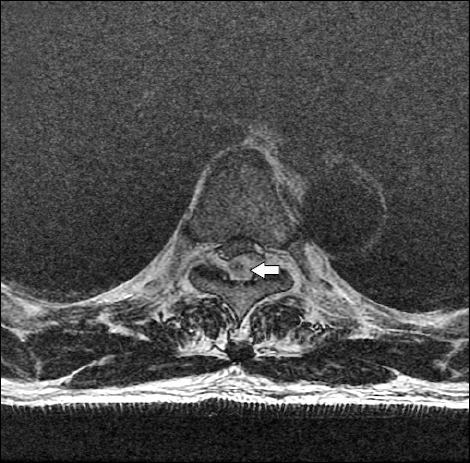
Fig. 2
Transaxil T2-weighted image. Nodular enhancing foci (white arrow) is noted inside the epidural hematoma at the level of T5 (right side).
For appropriate treatment of iatrogenic spinal epidural hematoma, we requested joint treatment with neurosurgery. At first, emergency surgery was considered. However, the patient and his family did not want surgery because of the risk of general anesthesia and surgery due to old age. After performing consultation with neurosurgeons and obtaining consent from the patient, we decided to use a less invasive method than surgery to reduce the pressure through drainage using an epidural needle. In a previous case report, epidural abscess has been drained using epidural catheter. Therefore, we thought that needle drainage might be possible because the hematoma was at an acute phase with fresh blood that had not yet been clotted.
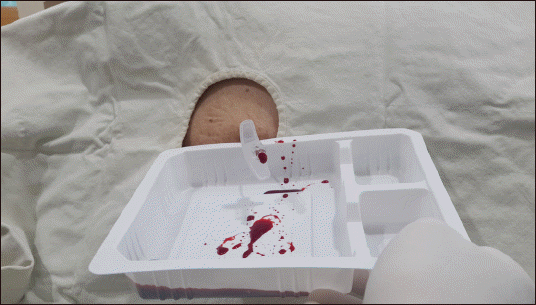
The procedure was performed on the patient’s left lateral position as before with an 18-gauge Tuohy needle inserted into the T4-5 level guided by fluoroscopy. Blood flowed out through the needle little by little. About 20 cc of blood was drained, similar to the amount expected by MRI (Fig. 3). The patient’s back pain was reduced after the treatment. However, there was no significant difference in the degree of motor function. The next day, the patient was transferred to neurosurgery and treated conservatively. His strength-lacking symptoms of the limbs were gradually improved. The patient was able to move by himself. After removing the foley catheter, the patient recovered to a state of spontaneous urination. He was discharged after 12 days of hospitalization. The patient was admitted to the hospital one week later and confirmed that his motor function was completely recovered. We recommended follow-up MRI to the patient. However, the patient refused because of the cost.
DISCUSSION
Epidural nerve block for post-herpetic neuralgia is very effective for pain control. It can be safely used. However, on rare occasion, it can lead to serious neurological complications. Despite several attempts have been to quantify the occurrence of epidural hematoma formation with improvements in our knowledge of risk factors and best management practices, its true incidence is difficult to determine. The incidence of epidural hematoma has been traditionally estimated at 1:15,000 in patients receiving epidural blockades [2]. In some cases, complications can result in permanent damage such as paraplegia at a rate of 0.1–1/10,000 [3]. Research on the prevalence of epidural hematoma is currently insufficient, although epidural hematoma has been reported in a variety of cases. The incidence of epidural hematoma has been calculated to be about 1 in 190,000 patients who received epidural anesthesia in a retrospective analysis [4]. In a 54-year retrospective review conducted in China, the incidence of epidural hematoma after neuraxial blockade has been found to be 2.14 of 100,000 [5]. These complications mainly occur in patients being administered with anticoagulants or those with have coagulation disorders. They rarely appear in healthy patients. According to Vandermeulen et al. [6], among 61 patients whose epidural hematomas were reported after epidural nerve block, 87% of these patients had coexisting coagulopathy while the remaining 13% of patients had no coexisting coagulopathy.
Clinical symptoms of epidural hematoma usually start with severe acute spinal pain with radiating pain appearing in associated limbs. These neurological symptoms can rapidly deteriorate with various degrees of motor and sensory nerve deficits as well as urination disorders [3]. In addition, symptoms of patients are very diverse. Therefore, when an unexpected situation occurs, it is important to be suspicious of epidural hematoma and approach accordingly.
In order to have a rapid and appropriate treatment, an accurate diagnosis has to be the priority. This is possible through examinations such as computed tomography (CT), myelogram, and MRI. With CT, it is difficult to distinguish epidural hematoma from other diseases that put pressure on the spinal dura mater. With myelogram, it is able to find the shape, size, and location of tumors. However, it is invasive. With MRI, it is able to discern subdural or subarachnoid hemorrhages, disc herniations, spinal cord edema, and so on in spinal cord injured patients with neurological manifestations. It is also non-invasive. It determines hematoma and nerve compression level, and also shows soft tissue damage around the lesion and the state of the spinal cord. Therefore, MRI has been considered to be the best diagnostic method [7]. For epidural hematoma treatment, surgical treatment is the top standard of treatment to remove hematomas through laminectomy. In cases where neurological symptoms are accompanied, a quick surgery after its onset can lead to good prognosis. It is believed that immediate decompression within 36 hours is linked to good prognosis [8]. However, according to Cai et al. [9], among 16 patients who acquired Brown-Séquard syndrome due to spontaneous epidural hematoma, 3 patients who received conservative treatment fully recovered. When compared to surgical treatment, conservative treatments were not related to bad neurological prognosis. Duffill et al. [10] have also shown good neurological prognosis without surgical treatment in 4 cases. They displayed improvement over time for their severe neurological symptoms caused by spontaneous epidural hematoma. Surgical decompression is the most fundamental treatment for removing epidural hematoma. However, surgery performed under general anesthesia can be invasive and dangerous for patients who are old or in poor general health. There might be concerns when awaiting natural mitigation from conservative treatments when conditions of continual neurological symptoms are present. Among different treatments for epidural hematoma, a less invasive decompression method than surgery called percutaneous needle aspiration has been reported for a few cases [11-14].
Levy [11] reported that, when blackish liquid blood (12–15 ml) was removed from a lumbar subdural hematoma in an 81-year-old woman who had severe back pain through a fluoroscopically guided lumbar puncture, the patient’s symptoms were almost immediately relieved. In the case report of Tabo et al. [12], two cases of epidural abscess occurred in patients who were treated at a pain clinic. In these cases, each abscess was removed through a method in which yellow pus was drained through 3 Tuohy needles and a catheter and washed with saline solution. Lyu et al. [13] have also reported that they have treated a 48-year-old man with severe pain who had walking and dysuria disorders by removing an epidural abscess with a needle guided by CT. In addition, after 30 cc of blood was removed from intraspinal hematoma after laminectomy using an 18-gauge Tuohy needle and catheter guided CT, complete resolution was achieved based on MRI and the patient was fully recovered in another study [14].
In our case, acute thoracic epidural hematoma was quickly diagnosed through MRI in a patient who had complications after a thoracic epidural nerve block. It was determined that decompression of the hematoma was required due to continual neurological symptoms.
In this patient, a nodular enhancing foci suspected of bleeding focus was noted inside the epidural hematoma on MRI. It was noticed less than 24 hours after the epidural block. For this reason, the epidural hematoma was considered to be at acute hemorrhage state in which the clot had not yet progressed. We thought that it might be possible to drain the hemorrhage through needle.
In a previous review for MRI findings of spinal hematoma, hyperacute hemorrhage was demonstrated to be iso- to hypo- intense on T1 weighted images and hyperintense on T2- weighted images due to fresh oxygenated blood [15]. This seemed to be similar to MRI findings in this case. In fact, fresh blood was drained when 18-gauge Tuohy needle was inserted.
Our case report suggests that it is possible to use decompression through a less invasive method for an acute epidural hematoma and observe its progress rather than performing immediate surgery. In addition, even if surgery is inevitable, such decompression method might make time for operation.
Such treatment might be recommended when hematoma is considered to be at acute phase based on patient history and MRI findings and when patient has no risk factor for hematoma. Although we performed drainage successfully with C-arm guide in this case, it might be better to perform it under CT guidance for patient safety.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download