This article has been
cited by other articles in ScienceCentral.
Abstract
Background:
The addition of fentanyl or epinephrine to bupivacaine enhances the quality of intraoperative spinal anesthesia during cesarean section. This study aimed to evaluate the beneficial effects of adding fentanyl or epinephrine to bupivacaine in spinal anesthesia solutions used for patients undergoing cesarean section.
Methods:
This retrospective study included 391 patients who underwent cesarean section under spinal anesthesia between March 2009 and February 2014. Parturients were categorized into group N (no addition; n = 103), group E (addition of epinephrine; n = 196), and group F (addition of fentanyl; n = 92). Perioperative hemodynamic changes, complications, sensory recovery times, Apgar scores, and cord blood pH were analyzed.
Results:
Nausea and vomiting occurred more frequently in group E than in the other two groups (P < 0.001 and P = 0.027, respectively). The mean sensory recovery times to T10 level showed statistically significant intergroup differences (P < 0.001). Group F showed the highest 1-min and 5-min Apgar scores, with statistically significant differences amongst the three groups (P = 0.007 and P < 0.001, respectively). However, the blood gas analysis variables of the cord blood did not show significant differences.
Conclusions:
Addition of fentanyl to bupivacaine was related to a longer sensory recovery time than did the addition of nothing or epinephrine. Moreover, it had been associated with beneficial effects such as a reduction in complications following spinal anesthesia.
Go to :

Keywords: Cesarean section, Epinephrine, Fentanyl, Spinal anesthesia
INTRODUCTION
The anesthetic technique used for cesarean delivery depends on the indication of cesarean section, emergency level, maternal hemodynamic status, and maternal preference [
1]. The use of general anesthesia for cesarean delivery has been declining over the recent years, and regional techniques are being increasingly recommended in obstetric anesthesia because of the absence of systemic drug effects on the mother and fetus [
2]. The addition of epinephrine to bupivacaine during spinal anesthesia for cesarean section has improved the quality of subarachnoid block [
3]. Furthermore, the addition of opiates to bupivacaine given for the same surgical procedure improves operative analgesia and provides extended postoperative pain relief [
4-
8].
Therefore, in the present retrospective study, we analyzed the medical records of patients who underwent cesarean delivery under spinal anesthesia with fentanyl or epinephrine added to the spinal solution to investigate differences in the quality of anesthesia and effects on newborns.
Go to :

MATERIALS AND METHODS
The study protocol was approved by the Institutional Review Board (IRB No. 2014-12-002).
Patient population
The parturients in the present study included patients who underwent cesarean delivery under spinal anesthesia at a single university hospital over a period of 5 years between March 2009 and February 2014. Among 587 patients, 391 were enrolled in the study after excluding those who gave birth to newborns with gestational age < 34 weeks (n = 55), gave birth to a fetus with an Apgar score of 0 who could not be resuscitated (n = 1), gave multiple births (n = 115), or had missing records (n = 25) (
Fig. 1). The parturients were classified into group N if nothing was added to the local anesthetic (n = 103), group E if epinephrine was added (n = 196), and group F if fentanyl was added (n = 92).
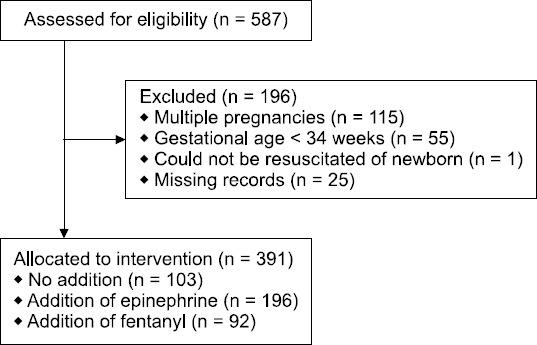 | Fig. 1
|
Anesthetic regimen
All parturients were admitted to the operating room without premedication; they underwent noninvasive blood pressure monitoring, electrocardiography, and pulse oximetry. Hemodynamic parameters were recorded every minute until delivery, every 3 min until the end of surgery, and every 5 min in the recovery room. After measuring the initial vital signs, Ringer’s lactate solution and oxygen were administered. Spinal anesthesia was induced at the L4–5 or L3–4 intervertebral spaces by adding 100–200 μg epinephrine (group E), 15 μg fentanyl citrate (group F), or nothing (group N) to 8–10 mg bupivacaine HCl (AstraZeneca, Australia). Left uterine displacement was maintained using a wedge under the right hip. Surgery was started when bilateral sensory blockade was achieved up to at least the T6 dermatome.
Measurements
Once the parturients were confirmed stable following anesthetic induction, the end-tidal carbon dioxide (ETCO2) was monitored. Apnea was suspected when ETCO2 was not detected for 20 s or more during surgery. The level of sensory block to cold sensation was checked using an alcohol swab at 1, 3, and 5 min after anesthetic induction. If the sensory block had not reached the T6 dermatome, additional checks were made every 2 min before surgery. After the surgery ended, the sensory block was checked every 10 to 15 min. When systolic blood pressure (SBP) was less than 100 mmHg more than two times, 5 mg of ephedrine or 50 μg of phenylephrine was administered intravenously. Fentanyl citrate was administered intravenously when pain control was needed.
The neonatal condition was evaluated by using 1-min and 5-min Apgar scores and arterial umbilical blood gas analysis.
Data collection
One researcher retrospectively reviewed the electronic medical records. These records provided all of the data required for the analyses.
For the comparison of hemodynamic changes, the initial vital signs of the patient prior to anesthesia induction were used as the baseline for vital signs recorded at 5, 10, 15, and 20 min after anesthesia induction. The block level was measured as the level of sensory block reached within 5 min after injection of the local anesthetic. The I-S time and S-D time were measured as the time from anesthetic induction to the start of surgery and from the start of surgery to delivery, respectively. The I-R time was measured as the time from anesthesia induction to sensory recovery to the T10 level after spinal anesthesia. Assessment of adverse events occurring after spinal anesthesia was performed by reviewing information inputted into the anesthesia record form. Adverse events other than pain and hypotension were reviewed from anesthetic induction to the end of surgery. Parturients were assessed to have pain if they had an intraoperative visual analogue scale score of more than 5 and received an additional intravenous analgesic (fentanyl) from delivery to the end of surgery. In addition, the number of fentanyl administrations and the total dosage of fentanyl were compared among groups. Parturients were assessed to have hypotension if they received a vasopressor (ephedrine or phenylephrine), according to the criteria mentioned in the Methods section (Measurements subsection) from anesthetic induction to expulsion of placenta.
The Apgar scores were determined using the information recorded by the neonatal nurse present in the operating room during delivery. Acidosis was assessed using the results of the blood gas analysis performed on the cord blood samples collected during surgery.
Hypotension can cause symptoms such as nausea and vomiting in the parturient, and acidosis in the fetus. Therefore, an additional analysis was performed to identify the relationship of hypotension with other variables.
Statistical analysis
Statistical analyses were performed using STATA (StataCorp, USA). Data are reported as means ± SD for continuous variables, and percentages for quantitative variables. Analysis of variance (ANOVA) test, Chi-squared test, or Fisher’s exact test was used depending on whether the variables were continuous or categorical. For example, the P values of the Kolmogorov–Smirnov test for patient’s age, height, and weight were 0.260, 0.200, and 0.200, respectively. Therefore, these continuous variables were analyzed by the ANOVA test. On the other hand, the incidence of emergency cesarean section and adverse events were analyzed by the Chi-squared test. The level of sensory block was analyzed by the Fisher’s exact test. Post-hoc analysis was performed using the Scheffe’s method. P values less than 0.05 were considered to indicate statistical significance.
Go to :

RESULTS
No significant intergroup differences were observed with respect to the variables age, height, and weight of the patient; gestational age; or the nature of surgery (i.e., emergency surgery) (
Table 1). The heart rate, and SBP diastolic blood pressures showed no significant interactions amongst three groups (P = 0.361, P = 0.569, and P = 0.897, respectively). The level of sensory block, I-S time, and S-D time were not significantly different amongst the three groups. The I-R time were significantly different amongst the three groups (P < 0.001) (
Table 2). Scheffé’s post-hoc analysis also showed significant differences between the groups (Group N vs. Group E, P < 0.001; Group N vs. Group F, P < 0.001; and Group E vs. Group F, P < 0.001).
Table 1
Demographic Data on Parturient Characteristics
|
Group N (n = 103) |
Group E (n = 196) |
Group F (n = 92) |
P value |
|
Age (yr) |
33.7 ± 4.6 |
33.9 ± 4.1 |
34.3 ± 4.5 |
0.567 |
|
Height (cm) |
160.1 ± 5.4 |
160.0 ± 5.8 |
159.4 ± 5.9 |
0.577 |
|
Weight (kg) |
70.7 ± 11.3 |
71.2 ± 11.9 |
69.3 ± 8.6 |
0.317 |
|
Gestational age (weeks) |
38.2 ± 1.5 |
38.0 ± 1.4 |
37.7 ± 1.3 |
0.059 |
|
Heart rate (beats/min) |
84.8 ± 16.2 |
84.5 ± 14.3 |
84.1 ± 12.4 |
0.361 |
|
Systolic blood pressure (mmHg) |
131.7 ± 20.7 |
128.2 ± 21.2 |
130.8 ± 26.5 |
0.569 |
|
Diastolic blood pressure (mmHg) |
77.8 ± 14.4 |
76.8 ± 14.0 |
77.4 ± 18.8 |
0.897 |
|
Incidence of emergency cesarean section (%) |
27.0 (26.2) |
57.0 (29.1) |
25.0 (27.2) |
0.872 |

Table 2
Variables Measured during the Intraoperative Period in Parturients Grouped according to the Agent Added to Local Anesthetic during Spinal Anesthesia
|
Group |
Mean (Median) |
SD |
SEM |
95% CI of difference |
P value |
|
|
|
[IQR] |
Lower |
Upper |
|
Block level (thoracic) |
N |
(6) |
[4–10] |
|
|
0.704 |
|
E |
(6) |
[4–10] |
|
|
|
|
F |
(6) |
[4–10] |
|
|
|
|
I-S time (min) |
N |
15.7 |
6.5 |
0.6 |
14.4 |
16.9 |
0.063 |
|
E |
14.7 |
5.5 |
0.4 |
13.9 |
15.5 |
|
|
F |
13.8 |
4.3 |
0.5 |
12.9 |
14.7 |
|
|
S-D time (min) |
N |
8.7 |
3.4 |
0.3 |
8.0 |
9.4 |
0.442 |
|
E |
8.7 |
3.5 |
0.3 |
8.2 |
9.2 |
|
|
F |
8.1 |
4.4 |
0.5 |
7.2 |
9.1 |
|
|
I-R time (min) |
N |
85.7 |
24.3 |
2.4 |
80.9 |
90.4 |
< 0.001 |
|
E |
97.1 |
26.6 |
1.9 |
93.3 |
100.8 |
|
|
F |
112.3 |
21.9 |
2.3 |
107.8 |
116.9 |
|

Among the adverse events following spinal anesthesia, nausea was most frequent in group E and least frequent in group F, and the frequency of nausea showed significantly different intergroup differences (Group N vs. Group E, P = 0.002; Group N vs. Group F, P = 0.169; and Group E vs. Group F, P < 0.001). Vomiting was most frequent in group E and least frequent in group F, and the frequency of vomiting showed significantly different intergroup differences (Group N vs. Group E, P = 0.045; Group N vs. Group F, P = 0.918; and Group E vs. Group F, P = 0.154). The frequency of apnea, pruritus, pain, and hypotension was not significantly different (
Table 3). However, there was a significant difference in the number of administrations of fentanyl for pain control (Group N vs. Group E, P = 0.164; Group N vs. Group F, P = 0.005; and Group E vs. Group F, P = 0.164). The total dosage of fentanyl administered for pain control was smallest in Group F (Group N vs. Group E, P = 0.048; Group N vs. Group F, P = 0.001; and Group E vs. Group F, P = 0.130).
Table 3
Comparison of the Prevalence of Spinal Anesthesia-related Adverse Events
|
Group N (n = 103) |
Group E (n = 196) |
Group F (n = 92) |
P value |
|
Nausea |
17 (16.5) |
67 (34.2) |
5 (5.4) |
< 0.001 |
|
Vomiting |
1 (1.0) |
14 (7.1) |
2 (2.2) |
0.023 |
|
Apnea |
NA |
NA |
NA |
NS |
|
Pruritus |
0 (0) |
3 (1.5) |
0 (0) |
0.435 |
|
Pain |
51 (49.5) |
78 (39.8) |
30 (32.6) |
0.052 |
|
Number of fentanyl administrations |
0.9 ± 1.1 |
0.7 ± 1.0 |
0.5 ± 0.8 |
0.005 |
|
Total fentanyl dosage (μg) |
47.8 ± 58.4 |
33.1 ± 48.5 |
20.6 ± 36.2 |
0.001 |
|
Hypotension |
86 (83.5) |
153 (78.1) |
66 (71.7) |
0.143 |

A comparison of the effects of the added agents on newborns showed no significant difference in acidosis assessed using cord blood analysis and the 5-min Apgar score. However, the 1-min Apgar scores showed significant differences (P = 0.009) (
Table 4). The analysis of the relationship of hypotension with other variables showed that 1-min and 5-min Apgar scores, HCO
3−, and actual base excess were significantly lower in parturients with hypotension than in parturients without hypotension, but nausea and vomiting were not significantly different (
Table 5). However, there was no significant difference in the pH, and the difference between the actual values was less than 0.1, indicating that the difference was probably not clinically relevant.
Table 4
Apgar Scores and Cord Blood Analysis of Newborns in the Three Groups
|
Group |
Mean |
SD |
SEM |
95% CI of difference |
P value |
|
|
Lower |
Upper |
|
Apgar 1 |
N |
6.9 |
0.9 |
0.9 |
6.7 |
7.1 |
0.009 |
|
E |
7.2 |
0.7 |
0.1 |
7.1 |
7.3 |
|
|
F |
7.2 |
0.8 |
0.1 |
7.1 |
7.4 |
|
|
Apgar 5 |
N |
8.6 |
0.8 |
0.1 |
8.4 |
8.7 |
0.055 |
|
E |
8.7 |
0.6 |
0.0 |
8.6 |
8.8 |
|
|
F |
8.8 |
0.5 |
0.0 |
8.7 |
8.9 |
|
|
pH |
N |
7.26 |
0.06 |
0.01 |
7.25 |
7.28 |
0.529 |
|
E |
7.27 |
0.06 |
0.01 |
7.26 |
7.28 |
|
|
F |
7.27 |
0.06 |
0.01 |
7.26 |
7.29 |
|
|
pO2
|
N |
16.9 |
5.1 |
0.5 |
15.8 |
17.9 |
0.394 |
|
E |
17.8 |
5.6 |
0.4 |
17.0 |
18.6 |
|
|
F |
17.6 |
4.8 |
0.5 |
16.6 |
18.6 |
|
|
pCO2
|
N |
53.8 |
9.4 |
1.0 |
51.9 |
55.8 |
0.094 |
|
E |
53.4 |
8.5 |
0.6 |
52.2 |
54.7 |
|
|
F |
53.5 |
8.6 |
0.9 |
51.8 |
55.3 |
|
|
HCO3−
|
N |
19.6 |
2.1 |
0.2 |
19.2 |
20.0 |
0.424 |
|
E |
19.9 |
2.0 |
0.2 |
19.6 |
20.2 |
|
|
F |
20.0 |
1.9 |
0.2 |
19.6 |
20.4 |
|
|
Base excess |
N |
–4.1 |
2.9 |
0.3 |
−4.7 |
−3.5 |
0.222 |
|
E |
–3.7 |
2.5 |
0.2 |
−4.1 |
−3.4 |
|
|
F |
–3.7 |
2.4 |
0.3 |
−4.7 |
−3.2 |
|

Table 5
Relationships of Hypotension with Nausea, Vomiting, Apgar Scores, and Cord Blood
|
Hypotension |
Mean |
SD |
SEM |
95% CI of difference |
P value |
|
|
Lower |
Upper |
|
Nausea |
No |
0.2 |
0.4 |
0.0 |
0.1 |
0.3 |
0.299 |
|
Yes |
0.2 |
0.4 |
0.0 |
0.2 |
0.3 |
|
|
Vomiting |
No |
0.0 |
0.2 |
0.0 |
0.0 |
0.1 |
0.299 |
|
Yes |
0.0 |
0.2 |
0.0 |
0.0 |
0.1 |
|
|
Apgar 1 |
No |
7.3 |
0.6 |
0.1 |
7.1 |
7.4 |
0.039 |
|
Yes |
7.1 |
0.8 |
0.1 |
7.0 |
7.2 |
|
|
Apgar 5 |
No |
8.8 |
0.5 |
0.1 |
8.7 |
8.9 |
0.033 |
|
Yes |
8.6 |
0.7 |
0.0 |
8.6 |
8.7 |
|
|
pH |
No |
7.28 |
0.05 |
0.01 |
7.27 |
7.29 |
0.097 |
|
Yes |
7.27 |
0.06 |
0.00 |
7.26 |
7.27 |
|
|
pCO2
|
No |
53.6 |
7.3 |
0.8 |
52.0 |
55.2 |
0.945 |
|
Yes |
53.5 |
9.1 |
0.5 |
52.5 |
54.6 |
|
|
HCO3−
|
No |
20.3 |
1.7 |
0.2 |
19.9 |
20.7 |
0.022 |
|
Yes |
19.7 |
2.1 |
0.1 |
19.5 |
20.0 |
|
|
Base excess |
No |
–3.2 |
2.0 |
0.2 |
–3.5 |
–2.7 |
0.008 |
|
Yes |
–4.0 |
2.7 |
0.2 |
–4.3 |
–3.7 |
|

Go to :

DISCUSSION
The present study found that sensory recovery time was longer in the group with fentanyl added to bupivacaine than in the other groups, and the occurrence of adverse events following spinal anesthesia, such as nausea and vomiting, was less frequent in this group. Moreover, the Apgar scores of the newborns showed higher values in the group with fentanyl added, but cord blood analysis indicated no significant effect of the added fentanyl on acidosis in newborns.
When performing spinal anesthesia, adding agents to the local anesthetic can reduce the amount of local anesthetic, sustain its analgesic effect, and prolong anesthesia time [
9]. Addition of opioids to local anesthetics has been used for over 30 years to enhance analgesia and reduce the dose of local anesthetics [
6]. A previous study that compared the effects of differences in anesthesia induction speed showed that epinephrine induced anesthesia slower than morphine or a combination of epinephrine and morphine did [
9]. Another study demonstrated an increase in the spread of spinal block because of the addition of intrathecal fentanyl. However, in the present study, this increase in spinal block was not significant (
Table 2). The number of fentanyl administrations and the total dosage of fentanyl for adequate pain control were significantly lower in the group with fentanyl added than in the group with no added agent, suggesting that the addition of fentanyl may have beneficial effects on pain control during cesarean section. Moreover, the sensory recovery time was longer in group F. However, whether sensory recovery time was longer in the groups with added agents than in the group with no added agent is questionable because of an increase in the volume of the spinal solution itself. Nevertheless, the injected volumes in groups E and F were only 0.1–0.3 ml larger than the volume in group N. Such a small difference in volume should not affect the quality of the block [
10].
In another similar study, Yu et al. [
11] found that the group receiving added meperidine had a greater incidence of intraoperative nausea or vomiting than did the group receiving added saline. Farzi et al. [
12] found no statistically significant difference in the occurrence of nausea and vomiting, and concluded that their occurrence during the operation may be partly due to surgical manipulation and peritoneal stimulation. However, in our study, the fentanyl group had a lower incidence of these adverse events. Hypotension can cause symptoms such as nausea and vomiting [
13], and acidosis in the fetus can also be caused by hypotension in the mother [
14]. Accordingly, an additional analysis was performed (
Table 5). Therefore, the presence of nausea and vomiting appears to be associated with the use of additional agents. Nevertheless, the variables in the newborns are affected more by hypotension of the parturients than by the differences in the drugs used.
A limitation of the present study was that when monitoring the recovery level of parturients receiving spinal anesthesia, only the sensory recovery level was checked and the motor recovery level was not recorded. We also could not compare the extended postoperative pain relief according to each group. The Apgar scores and cord blood values of newborns showed statistical significance, but the differences were too minute to have any actual clinical implications. Because the value of newborns to leave a sequela was close to zero, indicating that the effect was probably not clinically relevant.
Despite these limitations, our findings clearly suggest that the addition of fentanyl to the spinal solution was related to longer sensory recovery time and caused fewer incidences of nausea and vomiting. In addition, we believe that adding a small amount of fentanyl while performing spinal anesthesia during cesarean delivery can also enhance the quality of the block.
Go to :

ACKNOWLEDGEMENTS
This work was supported by a research grant from Jeju National University Hospital in 2015.
Go to :

REFERENCES
1. BirBach DJ, Browne IM. Anesthesia for obstetrics. Miller’s Anesthesia. 7th ed. Miller RD, editor. Philadelphia: Churchill Livingston;2010. p. 2203–40.
2. Block A, Covino BG. Effect of local anesthetic agents on cardiac conduction and contractility. Reg Anesth Pain Med. 1981; 6:55–61.
4. Abouleish E, Rawal N, Rashad MN. The addition of 0.2 mg subarachnoid morphine to hyperbaric bupivacaine for cesarean delivery: a prospective study of 856 cases. Reg Anesth. 1991; 16:137–40. PMID:
1883770.
5. Hunt CO, Naulty JS, Bader AM, Hauch MA, Vartikar JV, Datta S, et al. Perioperative analgesia with subarachnoid fentanyl- bupivacaine for cesarean delivery. Anesthesiology. 1989; 71:535–40. DOI:
10.1097/00000542-198910000-00009. PMID:
2679237.
6. Weigl W, Bieryło A, Krzemień-Wiczyńska S, Mayzner-Zawadzka E. Comparative study of postoperative analgesia after intrathecal administration of bupivacaine with fentanyl or morphine for elective Caesarean section. Anestezjol Intens Ter. 2009; 41:28–32. PMID:
19517674.
7. Abboud TK, Dror A, Mosaad P, Zhu J, Mantilla M, Swart F, et al. Mini-dose intrathecal morphine for the relief of post-cesarean section pain: safety, efficacy, and ventilatory responses to carbon dioxide. Anesth Analg. 1988; 67:137–43. DOI:
10.1213/00000539-198802000-00006. PMID:
3277478.
8. Chadwick HS, Ready LB. Intrathecal and epidural morphine sulfate for post-cesarean analgesia--a clinical comparison. Anesthesiology. 1988; 68:925–9. DOI:
10.1097/00000542-198806000-00015.
9. Abouleish E, Rawal N, Tobon-Randall B, Rivera-Weiss M, Meyer B, Wu A, et al. A clinical and laboratory study to compare the addition of 0.2 mg of morphine, 0.2 mg of epinephrine, or their combination to hyperbaric bupivacaine for spinal anesthesia in cesarean section. Anesth Analg. 1993; 77:457–62. DOI:
10.1213/00000539-199309000-00007. PMID:
8368545.
10. Denson DD, Bridenbaugh PO, Turner PA, Phero JC, Raj PP. Neural blockade and pharmacokinetics following subarachnoid lidocaine in the rhesus monkey I. Effects of epinephrine. Anesth Analg. 1982; 61:746–50. DOI:
10.1213/00000539-198209000-00006. PMID:
6896608.
11. Yu SC, Ngan Kee WD, Kwan AS. Addition of meperidine to bupivacaine for spinal anaesthesia for Caesarean section. Br J Anaesth. 2002; 88:379–83. DOI:
10.1093/bja/88.3.379.
12. Farzi F, Mirmansouri A, Forghanparast K, Heydarzadeh A, Abdollahzadeh M, Jahanyar Moghadam F. Addition of intrathecal fentanyl or meperidine to lidocaine and epinephrine for spinal anesthesia in elective cesarean delivery. Anesth Pain Med. 2014; 4:e14081. DOI:
10.5812/aapm.14081.
13. Chaudhari LS, Kane DG, Shivkumar B, Kamath SK. Comparative study of intrathecal pethidine versus lignocaine as an anaesthetic and a postoperative analgesic for perianal surgery. J Postgrad Med. 1996; 42:43–5. PMID:
9715298.
14. Ngan Kee WD, Lee A. Multivariate analysis of factors associated with umbilical arterial pH and standard base excess after Caesarean section under spinal anaesthesia. Anaesthesia. 2003; 58:125–30. DOI:
10.1046/j.1365-2044.2003.02888.x.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download