This article has been
cited by other articles in ScienceCentral.
Abstract
We report successful resuscitation of a patient after cardiac arrest on postoperative day 4 after coronary artery bypass grafting (CABG). The patient underwent proximal right coronary artery stent insertion 1 year preceding his CABG, and in-stent restenosis of the stent was found on coronary angiography (CAG). CABG was planned. The patient was treated with a nitroglycerin (NTG) for chest pain, and in the holding area of the operating theater, his chest pain resumed during brief cessation of the NTG while changing the syringe pump. Intraoperatively, normal flow was confirmed at the graft site with flowmetry, while the patient received a NTG infusion. On postoperative day 4, the patient developed chest pain and a subsequent cardiac arrest. He was resuscitated with chest compressions alone, and emergent CAG was performed. It showed coronary artery spasm of the left anterior descending coronary artery, confirmed by provocation testing. The patient was discharged with symptoms well controlled on oral medications.
Keywords: Cardiac arrest, Coronary artery bypass grafting, Coronary artery spasm, Nitroglycerin, Percutaneous coronary intervention
INTRODUCTION
Coronary artery bypass grafting (CABG) remains an established form of treatment for coronary artery disease unresponsive to medical treatment or percutaneous coronary intervention. Even with a low overall mortality rate of approximately 2–3%, the postoperative period after CABG is a high-risk period [
1]. Coronary artery spasm (CAS) after CABG is relatively uncommon. However, without appropriate management, it may cause angina, malignant arrhythmias, myocardial infarction, or even cardiac arrest. We herein present a case of successful resuscitation of cardiac arrest caused by life-threatening CAS on postoperative day 4 after CABG.
CASE REPORT
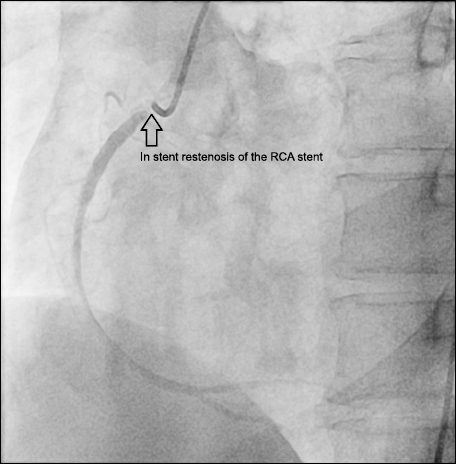
A 48-year-old male with hypertension, dyslipidemia, and a 30 pack-year history of smoking was referred to our hospital for CABG to treat unstable angina. The patient previously underwent stent insertion in the proximal right coronary artery (RCA) in 2015. Two months after the procedure, coronary angiography (CAG) was performed because of chest pain, which showed 70% stenosis of the RCA; another stent was subsequently inserted into the artery. The 1-year follow-up CAG revealed in-stent restenosis of the RCA stent (
Fig. 1), and the patient was transferred to our institution for CABG.
Fig. 1
Coronary angiography at another institution revealed in-stent restenosis of the previous right coronary artery (RCA) stent (blank arrow).
After arrival at our hospital, the patient underwent repeat CAG, which showed the same results as at the previous institution. The patient’s echocardiogram showed preserved left ventriclular systolic function, with a 53% ejection fraction and hypokinesia of the basal inferoseptal wall. His electrocardiogram (ECG) exhibited normal sinus rhythm, with no evidence of ST depression. His troponin I and creatine kinase-MB isoenzyme (CK-MB) values were normal (0.007 ng/ml and 0.18 ng/ml, respectively), but his pro-brain natriuretic peptide level was slightly increased (94.93 pg/ml).
Upon admission to our institution, the patient was initially treated with 0.5 μg/kg/min nitroglycerin (NTG) for his chest pain. Despite this, the pain worsened and the NTG was increased to 1 μg/kg/min. Considering his high dependency on NTG, the decision was made to proceed with urgent CABG surgery. In the holding area outside the operating theater, the NTG was stopped for a few seconds to change the syringe pump; this caused immediate exacerbation of the patient’s chest pain. Resumption of the infusion led to dramatic pain relief. The ECG showed normal sinus rhythm, without ST changes, throughout this whole sequence of holding room events.
After the patient was transferred to the operating theater, routine monitors were applied, including a radial artery catheter for invasive blood pressure measurements. Anesthesia was induced with midazolam 2 mg followed by continuous infusions of propofol, remifentanil, and rocuronium. Off-pump CABG was performed, with the right internal thoracic artery connected to the distal RCA. NTG was stopped for a few minutes and volume was infused to optimize hemodynamic status after the grafted vessel was perfused. At that time period, the patient’s systolic blood pressure, heart rate, cardiac output, cardiac index, pulmonary artery wedge pressure, and pulse pressure variation were 80–90 mmHg, 85–90 beats/min, 4.3 L/min, 2.5 L/min/m2, 4 mmHg, and 12%, respectively. However, flowmetry showed no flow at the graft site and new ST depression was evident on the ECG. NTG was restarted to improve perfusion of the coronary artery, and 1 μg/kg/min dobutamine was infused to maintain the blood pressure. The ST depression improved with these infusions, and the surgeons reexamined the operative field. The surgeons suspected failure of the graft so decided to revise the graft. After reinserting the graft at another site in the RCA, the NTG was resumed and more than 40 ml/min flow was confirmed at the new graft site.
The patient was transferred to the intensive care unit and extubated 4 hours after surgery. His vital signs were stable, with a systolic blood pressure of 120–150 mmHg. Six hours after surgery, the NTG was tapered off, and refractory chest pain combined with sweating appeared 3 hours later. A 0.5 μg/kg/min NTG infusion was again begun. The next day, the NTG was tapered off, and the patient was transferred to the general ward, with his symptoms under control.
When the surgeons were conducting their morning rounds on postoperative day 4, the patient complained of refractory chest pain similar to that experienced preoperatively. He then went into cardiac arrest, with pulseless electrical activity noted on the ECG. The patient was successfully resuscitated after 2 minutes of cardiac compressions, before any medications were administered. ST depression in leads II and aVF of the 12-lead ECG spontaneously improved a few minutes after the patient’s resuscitation. Emergent CAG showed a patent RCA graft site but diffuse luminal irregularity of the left anterior descending artery (
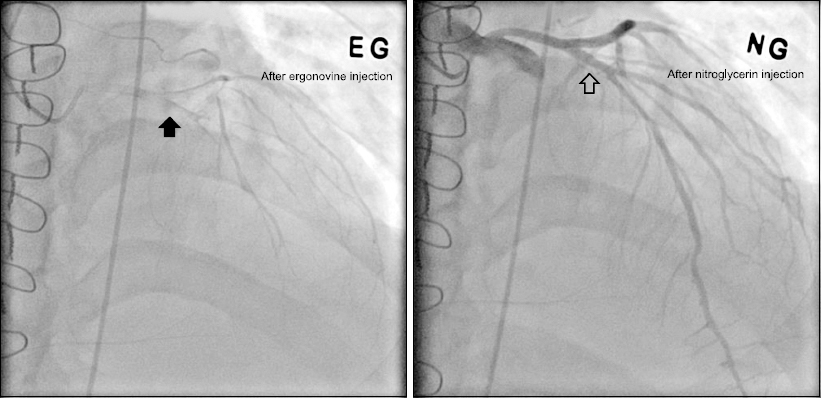
Fig. 2). A spasm provocation test with ergonovine was performed, which showed total occlusion with thrombolysis in myocardial infarction 0 flow at the proximal left anterior descending coronary artery, as well as relief of the obstruction with intracoronary NTG injection (
Fig. 3). This confirmed the diagnosis of CAS.
Fig. 2
Postoperative emergent coronary angiography showed diffuse luminal irregularity of the left anterior descending artery (black arrow) and a patent right coronary artery graft (blank arrow).
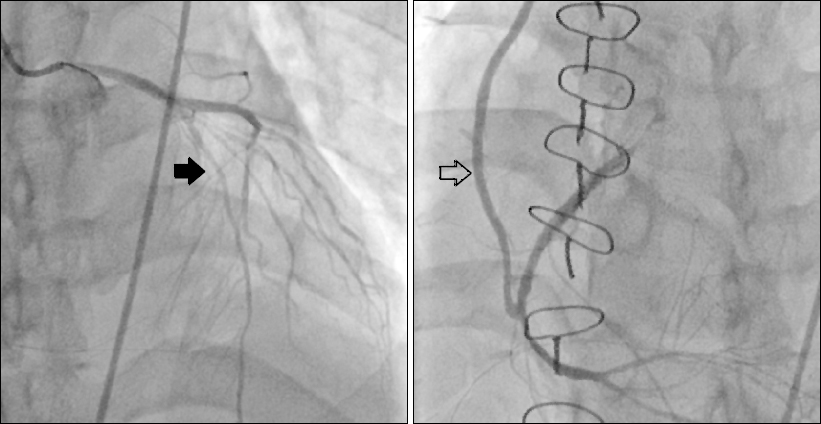
Fig. 3
In the ergonovine provocation test, spasm of the left ascending artery (black arrow) was relieved by intracoronary nitroglycerin injection (blank arrow), thereby confirming the diagnosis of coronary artery spasm.
The patient was transferred to the coronary care unit, where he was treated with a 5 μg/min NTG infusion and 90 mg of oral diltiazem. His peak troponin I level was 6.609 ng/ml on postoperative day 2, which subsequently decreased. His peak CK-MB was 24.97 ng/ml on postoperative day 1, which normalized by postoperative day 3. Four days after being diagnosed with CAS, the patient was discharged for the hospital, with his symptoms well controlled on diltiazem, isosorbide, trimetazidine, aspirin, clopidogrel, and atorvastatin.
DISCUSSION
Cardiac arrest shortly after CABG can have many causes. Myocardial infarction is relatively common among patients who sustain a cardiac arrest after CABG. Despite technical advances in CABG during recent years, perioperative myocardial ischemia associated with increased morbidity and mortality occurs with an incidence of 3.5–10% [
2]. Myocardial infarction appearing shortly after CABG may be the result of CAS, incomplete revascularization caused by graft failure, or stenosis or occlusion of a native coronary artery.
CAS may cause a variety of symptoms. When it persists for a sufficiently long period of time, it can lead to a wide spectrum of myocardial ischemic manifestations, including silent myocardial ischemia, stable angina, acute coronary syndrome, and sudden death [
3-
5]. Brief episodes of CAS after CABG are usually confined to a single coronary artery or graft and go unnoticed, as they often produce minimal symptoms, but serious outcomes including death have been reported [
6]. The incidence of CAS after CABG is reported to be 0.8–1.3% [
7,
8]. Predisposing factors, including vascular damage, high levels of endogenous or administered vasoconstrictors, oxidative stress, vascular injury during intraoperative manipulation, platelet activation at the vascular endothelial injury site, and hypothermia, are poorly understood and the contributing roles of these factors have not been firmly established.
Most CAS after CABG occurs within the first 8 hours (range, 3 to 8 hours; mean, 5.6 hours) [
6]. This case is unusual in that CAS occurred 4 days after CABG. Considering that the patient’s perioperative symptoms were highly dependent on NTG, the possibility of misdiagnosed preoperative CAS aggravated by surgery cannot be completely excluded. Successful treatment with NTG and calcium channel blocker infusion has been reported in a patient with undiagnosed variant angina during surgery [
9]. In our patient, in whom intraoperative ST depression was improved with a NTG infusion, it is possible that the lack of flow noted at the first graft site was caused by CAS.
Another possible etiology of CAS in this case involves the prior stent insertion. A review by Brott et al. [
10] described coronary spasm after drug-eluting stent placement, with poor outcomes in diffuse severe spasm. The pathophysiologic mechanisms remain unclear but may involve medications released from the stent, delayed endothelialization after stent placement, and the polymer coating on the stents [
11].
In the present case, graft failure was ruled out by CAG, and CAS was diagnosed by ergonovine provocation testing. The patient responded to conventional medical treatment of CAS. In patients highly dependent on NTG, CAS should always be considered, even if there appears to be an obvious coronary artery lesion.
In conclusion, this case illustrates how provocative CAS testing was very helpful to prevent another emergency operation for recurrent postoperative chest pain. Although the case was complicated because previous procedures were performed in another hospital, the possibility of variant angina and the use of a calcium channel blocker infusion should have been considered [
12].





