INTRODUCTION
The transrectal ultrasound-guided prostate biopsy (TRUS-PBx) is one of the most common procedures used for diagnosing prostate cancer, with approximately 800,000 biopsies performed annually in the United States [
1]. Many patients recognize the TRUS-PBx as a traumatic and worrisome experience because of the fear of cancer diagnosis, examination of a sexual organ through the anus, and anticipated pain [
2]. Indeed, 64–96% of patients undergoing TRUS-PBx complained of discomfort, with 12–22% of patients complaining of moderate to severe pain [
3]. However, the psychological stress, pain, and discomfort of patients have been largely unaddressed or overlooked.
Inadequate or lack of anesthesia may cause undue discomfort or injury to the patient due to poor cooperation, and adverse physiologic effects or psychological stress. In addition, patient movement during the procedure may result in the failure to accurately locate suspicious areas or in the biopsy needle missing its intended target. The performance of TRUS-PBx without discomfort allows a greater number of samples to be collected leading to a higher biopsy success rate. It is now well accepted that some form of anesthesia should be administered when performing TRUS-PBx [
4].
Although various anesthetic methods for TRUS-PBx have been investigated [
2,
4,
5], there is no consensus regarding an optimal anesthetic technique. Deep sedation can achieve sufficient anxiolysis and prevent movement during the procedure, but may also cause adverse side effects. Sedation using propofol is known to promote intraprocedural comfort and increase patient satisfaction [
6]; however, sedation using only propofol is unable to completely control all levels of pain intensity during TRUS-PBx [
7]. Despite this, some clinicians have suggested that the intensity of pain during TRUS-PBx is tolerable [
8], and it remains unverified whether analgesics are required in combination with propofol deep sedation during TRUS-PBx.
Meperidine, an opioid analgesic, has been frequently administered in conjunction with sedation during invasive diagnostic and therapeutic procedures due to its relatively short duration of action and it having less cardiorespiratory depressant effects than other potent opioids [
9].
In this study, we attempted to investigate if meperidine is required to improve the intraprocedural quality of sedation using bolus propofol administration and to reduce postprocedural pain and discomfort associated with TRUS-PBx.
Go to :

MATERIALS AND METHODS
Study population
The study protocol was approved by our Institutional Review Board and is listed on a World Health Organization recognized registry. After receiving written informed consent, 30–80 years old male patients with an American Society of Anesthesiologists (ASA) physical status of class I or II, that were scheduled to undergo TRUS-PBx in the sedation clinic of our hospital, were included in this prospective, randomized double-blind study.
The exclusion criteria included the use of monoamine oxidase inhibitors and/or antidepressant drugs, chronic opioid use, an allergy to propofol and/or meperidine, and the presence of any of the following medical conditions: neurological or psychiatric disease, sleep disorder, active anal disease (such as acute anal fissure), prostatitis, chronic obstructive lung disease, asthma, uncontrolled hypertension, renal dysfunction, chronic liver disease, thyroid disorder, and bleeding diathesis. Withdrawal criteria included poor patient compliance that prevented completion of the procedure and prolonged procedure time (> 20 minutes).
Study protocol
All patients were admitted to the day care center in the morning of the procedure and received a 1.0 g intravenous (i.v.) dose of prophylactic ceftizoxime prior to the biopsy. The patients’ names and group allocation were coded, and this information remained concealed until completion of the statistical analyses. Randomization was performed using a block randomization technique, with opaque sealed envelopes, by an anesthetist who did not participate in the sedation of the patients or the data collection. Random numbers were grouped in blocks in an allocation ratio of 1 : 1 for the two study groups. Nurses who were not involved in this study in any other capacity prepared the drugs for administration. Prior to the procedure, the age, weight, height, body mass index, ASA class, prostate specific antigen (PSA) value, previous experience with TRUS-PBx, and history of anal disease of each patient were evaluated.
Upon arrival to the sedation clinic, routine monitoring and bispectral index (BIS) devices were applied to the patient, then i.v. meperidine 0.5 mg/kg (Group M) or i.v. normal saline (at the same volume as that of the meperidine; Group C) was injected by the first investigator. Oxygen at 5 L/min was administered via face mask to maintain the peripheral oxygen saturation (SpO2) > 95%. The patient was placed into the left lateral decubitus position and initial blood pressure (in the dependent arm), heart rate, and SpO2 were measured. Following the administration of an i.v. injection of 40 mg 1% lidocaine to prevent injection pain, sedation was induced with i.v. 1% propofol 1.5 mg/kg by the second investigator. The patient’s neck was extended slightly to maintain airway patency during sedation. If hypoxemia associated with respiratory depression (defined as SpO2 < 95%) occurred, the patient was treated with the jaw thrust maneuver and airway insertion; if hypoxemia continued, respiration was assisted via manual ventilation through a face mask. The number of patients presenting with hypoxemia was recorded. After confirming loss of the eyelash reflex, rectal cleansing with betadine and a digital rectal exam were completed. Then TRUS was performed using a 7 MHz mechanical probe (BK, Brüel and Kjaer, Denmark) coated with 2% lidocaine jelly. Biopsy samples were collected using an 18-gauge Trucut needle; all patients underwent a 12-core biopsy performed by the same urologist. Additional i.v. propofol 0.5 mg/kg was administered (by the second investigator) when a patient moved during the procedure or the BIS value increased to > 75. Both the second investigator and the urologist were blinded to the group allocation. Hypotension (defined as systolic blood pressure [SBP] of < 80 mmHg, or a 30% decrease in relation to the baseline value) and bradycardia (defined as heart rate [HR] of < 45 beats/min) were treated with 10 mg i.v. ephedrine and 0.5 mg i.v. atropine, respectively. The procedure and recovery from anesthesia was conducted in the fully-equipped sedation clinic, then the patients were transferred to the day care center after the recovery period.
Patient evaluations
We manually recorded the BIS value, SBP, diastolic blood pressure (DBP), HR, and SpO2 immediately after the position change to left lateral decubitus (T0), 5 minutes after the initial recording (T5), and at the end (Tend) of the procedure; total propofol dose used; and duration of the operation. Patient movement was evaluated to determine the quality of sedation and was graded as follows: 1, no movement; 2, minor movement not interfering with the procedure; 3, purposeful movement transiently interfering with the procedure; 4, purposeful movement that made the procedure difficult; and 5, requirement for supplementation with general anesthesia to complete the procedure.
The modified observer’s assessment of alertness/sedation score (MOASS) was determined immediately after the procedure (Tend) and was graded as follows: 1, not responsive to mild prodding or shaking; 2, responsive only after mild prodding or shaking; 3, responsive only after loud or repeated patient address; 4, lethargic response to patient’s name spoken in a normal tone; and 5, ready response to patient’s name spoken in a normal tone.
The incidence of postprocedural pain was determined using the pain intensity numeric rating scale (NRS); the patients were asked to rate their pain intensity from 0 (“no pain”) to 10 (“worst pain”) at 15 (R15), 30 (R30), 45 (R45), and 60 (R60) minutes post-procedure. The highest of the four NRS values for each patient was used to calculate the mean NRS score for each treatment group and to compare the two groups. The degree of pain experienced was interpreted as none (0), mild (1–3), moderate (4–6), or severe (> 7); an additional analgesic (30 mg of i.v. ketorolac tromethamine) was administered to patients who reported an NRS ≥ 4. In addition, patient BP, HR, and SpO2 were recorded at R15, R30, R45, and R60; the use of additional analgesics; and any drug- or procedure-induced side effects (nausea, vomiting, dizziness, and drowsiness) were noted.
Patient satisfaction and willingness to undergo repeat biopsies by the same method were also evaluated before discharge from the day care center. Patient satisfaction was graded as highly satisfactory, satisfactory, unsatisfactory, or very unsatisfactory. Intraprocedural and postprocedural variables were evaluated and recorded by a nurse who was blinded to the group allocation.
Statistics
The number of patients required in each group was determined after a power calculation based on data from a pilot study. We anticipated a difference of 30% in the incidence of postprocedural pain between the control and the meperidine group as being clinically meaningful. Therefore, we calculated that 27 patients were required in each group for a type I error of 0.05 and a type II error of 0.2. We increased the recruitment by 10% to compensate for unexpected loss. Statistical analyses were carried out using SPSS® statistical software, version 20.0 (IBM Corp., USA) for Windows®. Parametric variables were expressed as the mean ± SD. Age, weight, height, body mass index, PSA value, prostate volume, duration of procedure, total propofol dose used, mean NRS value, mean MOASS, BP, HR, SpO2, and BIS values were analyzed using the Student’s t-test. The χ2 test with Sheffé’s test was used for post-hoc comparison of the following data: ASA class, the number of patients with previous anal disease, experience with prostate biopsy, requirement of additional propofol doses, incidence of pain (including degree of pain), and side effects. Patient movement was analyzed by the Mann-Whitney test. A value of P < 0.05 was considered statistically significant.
Go to :

RESULTS
A total of 60 patients were enrolled in our study (
Fig. 1). Patient characteristics and periprocedural data were not significantly different between the two groups. However, the total propofol dose used was significantly lower in Group M than in Group C (P = 0.036), as demonstrated in
Table 1.
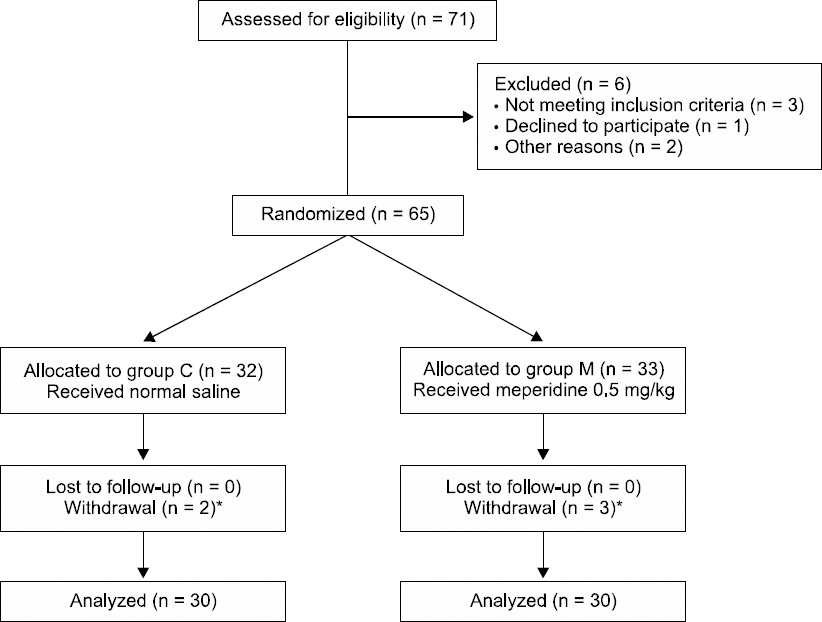 | Fig. 1CONSORT flow chart of patients enrolled in this study. *All withdrawals were due to the prolongation of the procedure (> 20 min). Group C: propofol group, Group M: propofol and meperidine group. 
|
Table 1
Patient Characteristics and Perioperative Data
|
Group C (n = 30) |
Group M (n = 30) |
P value |
|
Age (yr) |
68.2 ± 8.2 |
66.9 ± 8.0 |
0.571 |
|
Weight (kg) |
65.2 ± 8.5 |
67.7 ± 8.2 |
0.252 |
|
Height (cm) |
167.6 ± 5.4 |
168.1 ± 5.2 |
0.735 |
|
Body mass index (kg/m2) |
23.2 ± 2.5 |
23.9 ± 2.1 |
0.274 |
|
ASA physical status class I/II |
14/16 |
11/19 |
0.432 |
|
Prostate specific antigen (ng/ml) |
17.3 ± 27.5 |
16.3 ± 21.8 |
0.881 |
|
Initial/repeat biopsy |
23/7 |
25/5 |
0.519 |
|
Anal disease |
7 (23%) |
5 (16%) |
0.519 |
|
Estimated prostate volume (g) |
45.6 ± 20.9 |
50.7 ± 24.6 |
0.395 |
|
Duration of procedure (min) |
9.7 ± 2.2 |
9.9 ± 1.7 |
0.604 |
|
Total propofol dose used (mg) |
131.6 ± 25.8 |
118.1 ± 22.8 |
0.036 |
|
Propofol dose used (mg/kg) |
2.0 ± 0.4 |
1.75 ± 0.3 |
0.002 |
|
Additional propofol number of doses |
|
|
0.007 |
|
0 dose |
8 (26.7%) |
16 (53.3%) |
|
|
1 dose |
10 (33.3%) |
13 (43.3%) |
|
|
≥ 2 doses |
12 (40%) |
1 (3.3%) |
|

During the procedure, SBP, DBP, HR, SpO
2, and BIS values were not significantly different between the two groups (Figs.
2 and
3). Patient movement was not significantly different between the two groups (
Table 2), but additional propofol was administered to 22 patients in Group C (73.3%) compared to 14 patients in Group M (46.7%), which can be seen in
Table 1.
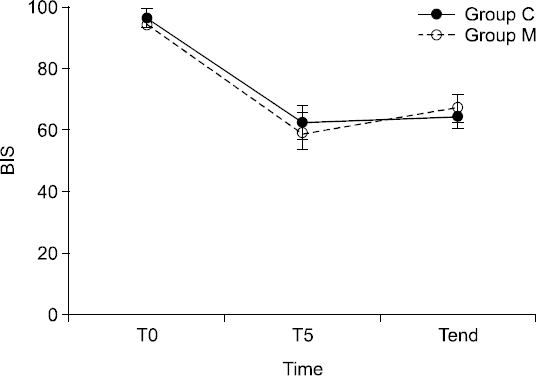 | Fig. 2Bispectral index during sedation. Data are presented as mean ± SD. T0: initial monitoring, T5: 5 minutes after initial monitoring, Tend: the end of the procedure. Group C: propofol group, Group M: propofol and meperidine group. 
|
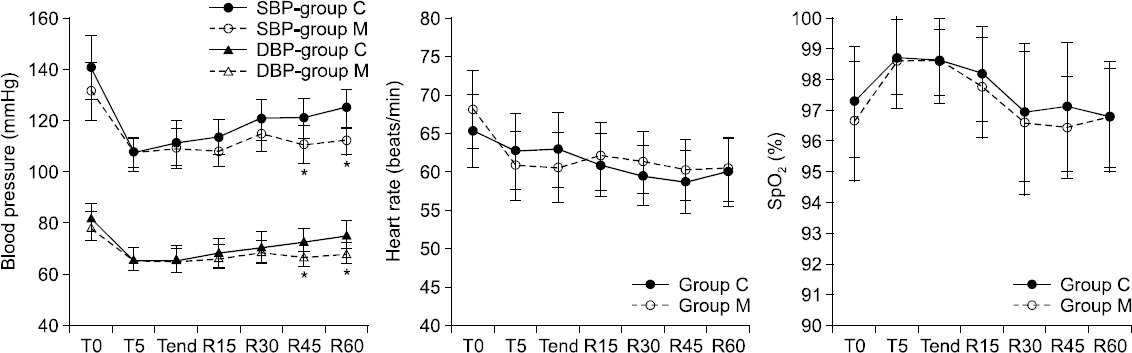 | Fig. 3Changes in blood pressure, heart rate, saturation during periprocedural periods. Data are presented as mean ± SD. Student’s t-test, *P < 0.05 vs group C, T0: initial monitoring, T5: 5 minutes after initial monitoring, Tend: the end of the procedure; R15, R30, R45, R60: 15, 30, 45, 60 minutes after the procedure, Group C: propofol group, Group M: propofol and meperidine group. SBP: systolic blood pressure, MBP: mean blood pressure, SpO2: peripheral oxygen saturation. 
|
Table 2
Patient Movement during the Procedure
|
Grades |
Group C (n = 30) |
Group M (n = 30) |
|
1 |
8 (26.7%) |
13 (43.3%) |
|
2 |
13 (43.3%) |
12 (40%) |
|
3 |
9 (30%) |
5 (16.7%) |
|
4 |
0 (0%) |
0 (0%) |
|
5 |
0 (0%) |
0 (0%) |

After the procedure, mean MOASS was not significantly different between the two groups (
Table 3; Student’s
t-test, P = 0.055). Postprocedural SBP and DBP were significantly lower in Group M than in Group C at R45 and R60 (SBP: P = 0.044 at R45 and P < 0.001 at R60; DBP: P = 0.014 at R45 and P = 0.006 at R60), but HR and SpO
2 were not significantly different between the two groups (
Fig. 3). No patients exhibited hypotension or bradycardia (and therefore, did not require ephedrine or atropine administration) throughout the periprocedural period. The mean NRS value during the 1 hour recovery period and the pain incidence were significantly lower in Group M than in Group C (P = 0.012), as seen in
Table 4. Administration of an additional analgesic (30 mg of i.v. ketorolac tromethamine) was required for 7 patients (23.3%) in Group C and 1 patient (3.3%) in Group M.
Table 3
Modified Observer’s Assessment of Alertness/sedation Score (MOASS) at the End of the Procedure
|
MOASS |
1 |
2 |
3 |
4 |
5 |
Mean MOASS |
|
Group C (n = 30) |
13 (43.3%) |
4 (13.3%) |
0 (0%) |
11 (36.7%) |
2 (6.7%) |
2.5 ± 1.5 |
|
Group M (n = 30) |
7 (23.3%) |
1 (3.3%) |
7 (23.3%) |
7 (23.3%) |
8 (26.7%) |
3.3 ± 1.4 |

Table 4
|
Group C (n = 30) |
Group M (n = 30) |
P value |
|
Mean NRS score |
1.5 ±1.9 |
0.4 ± 1.0 |
0.012 |
|
Total pain incidence |
14 (46.6%) |
6 (20.0%) |
0.028 |
|
Pain degree |
|
None (0) |
16 (53.3%) |
24 (80.0%) |
0.023 |
|
Mild (1–3) |
7 (23.3%) |
5 (16.7%) |
0.519 |
|
Moderate (4–6) |
7 (23.3%) |
1 (3.3%) |
0.052 |
|
Severe (≥ 7) |
0 |
0 |
N/A |

Three patients experienced respiratory complications during the periprocedural period (
Table 5). In Group C, the SpO
2 of one patient with chronic bronchitis decreased to 92% after induction, but recovered to 98% after the jaw thrust maneuver was performed. In another patient, the SpO
2 decreased to 89% during the recovery period, but increased to 95% after encouraging respiration. In Group M, one patient exhibited a decreased SpO
2 of 88% during the procedure resulting from upper airway obstruction due to retrognathia, but the SpO
2 increased to 99% after the jaw thrust maneuver and oropharyngeal airway insertion were performed. Other drug-induced complications such as nausea, vomiting, dizziness, and drowsiness did not occur in either group.
Table 5
|
Group C (n = 30) |
Group M (n = 30) |
P value |
|
Drug induced |
|
Respiratory depression |
2 (6.7%) |
1 (3.3%) |
N/A |
|
Procedure induced |
|
Tenesmus |
21 (70%) |
12 (40%) |
0.020 |
|
Rectal bleeding |
1 (3.3%) |
1 (3.3%) |
N/A |
|
Residual sensation of urine |
9 (30%) |
4 (13.3%) |
0.209 |

Tenesmus was significantly less frequent in Group M than in Group C (P = 0.020), as demonstrated in
Table 5. Two patients had rectal bleeding; the bleeding of one patient in Group C ceased after gauze compression, while a patient in Group M required vascular clamping of an anal vessel by colonoscopy. No patients required hospitalization due to complications. There was no significant difference in patient satisfaction between two groups (χ
2 test, P = 0.601). In Group C, 15 patients rated the procedure as “highly satisfactory” and the remaining 15 patients rated it as “satisfactory.” Eighteen patients in Group M rated the procedure as “highly satisfactory” and the remainder rated it as “satisfactory.” All patients, with the exception of one member of Group C, indicated that they would be willing to undergo a repeat biopsy using the same anesthetic method, if required.
Go to :

DISCUSSION
The aim of this study was to determine if pretreatment with meperidine is required with deep sedation for patients undergoing TRUS-PBx. We confirmed that meperidine pretreatment reduced the intraprocedural total propofol dose and the postprocedural pain and discomfort in patients undergoing deep sedation with bolus propofol for TRUS-PBx.
Among several anesthetic techniques, the periprostatic nerve block (PPNB) is generally considered to be the ‘gold standard’ for providing comfort and pain relief for patients undergoing TRUS-PBx [
5]. Nevertheless, the PPNB is only used clinically by 11% of urologists [
10] due to the need for multiple needle punctures, a mild stinging sensation, pain during injection, risk of infection, and risk of intravascular injection [
11-
13].
Sedation was suggested as an alternative method for minimizing patient anxiety, pain, and discomfort during TRUS-PBx [
6,
14]. The most commonly used sedatives are midazolam and propofol [
15,
16]; however, midazolam did not achieve deep sedation in most studies, and it is not easy to clinically adjust the level of sedation with bolus administration. TRUS-PBx is performed in a short period on an outpatient or day care center basis, and propofol is appropriate in such cases because it provides a good amnesia, an anxiolytic effect with rapid onset, rapid recovery, and has minimal side effects. Peters et al. [
17] observed that propofol bolus injections at a dose of 1–1.5 mg/kg significantly decreased patient discomfort and significantly increased patient satisfaction with the procedure. Park et al. [
16] proposed that conscious sedation at a MOASS level of 3 is appropriate for the patient and surgeon during TRUS-PBx, and that an effect-site propofol concentration of 1.5 μg/ml is an optimal dose. However, we considered deep sedation to be superior because conscious sedation (without analgesia) can result in procedure interruption. Unfortunately, many studies have not reported the total dose of propofol used, whether it was delivered by bolus or continuous infusion, the duration of anesthesia, or the quality of sedation [
7,
17,
18]. In a pilot study, we found that deep sedation could be induced in most patients with a bolus injection of i.v. propofol 1.5 mg/kg, and be maintained for an average of 5 minutes without severe respiratory depression. However, in most cases the procedure time was extended by more than 5 minutes, and patients required additional i.v. propofol 0.5 mg/kg upon movement. Although propofol is preferably administered by variable rate infusion, we chose the intermittent bolus injection method due to the short procedure time of TRUS-PBx.
In our study, the BIS value decreased to approximately 40–50 immediately after i.v. propofol injection, then increased to 62.5 in Group C and 59.6 in Group M five minutes after induction of sedation. Thereafter, the BIS was maintained at a level of 50–70. Nevertheless, 43% of the patients in Group C and 50% of the patients in Group M demonstrated a MOASS value of 4 or 5 immediately after the procedure, indicating that TRUS-PBx can be performed without prolonging deep sedation.
There are two main factors that contribute to pain during a TRUS-PBx are as follows: anal discomfort caused by ultrasound probe insertion, and penetration of the prostatic capsule by the biopsy needle [
2]. Although propofol sedation has advantages over a PPNB, it is unable to completely prevent intraprocedural patient movement and postprocedural pain due to lack of analgesic effect [
7]. Furthermore, pain during a TRUS-PBx was proven to be gradually accumulated from the first core biopsy to the last, even under anesthesia [
19]. Additional doses of propofol should be injected upon patient movement, and a high dose may be required. Therefore, propofol sedation should be administered with mild analgesics that have a propofol-sparing effect to prevent cardiorespiratory complications. Zisman et al. [
20] recommended the use of mild analgesics for 24 hours after biopsy, especially for patients who report severe intraprocedural pain, younger patients, and patients having inflammatory infiltrate. Likewise, Kang et al. [
18] demonstrated that propofol administered with remifentanil significantly reduced moderate to severe pain compared to 2% lidocaine gel. However, remifentanil is unsuitable for postprocedural pain control due to a rapid offset of analgesic effect after terminating the infusion. Barbosa et al. [
7] proposed that propofol 1.5 mg/kg administered with fentanyl 0.5 μg/kg, or with PPNB, is significantly more effective for pain control compared to propofol alone, although all approaches resulted in similar BIS values. However, in two cases of propofol administration with fentanyl, respiratory depression was observed, which was reversed by positive-pressure ventilation. Nishikawa et al. [
21] also reported that 47.5% of the patients that received propofol with fentanyl experienced hypotensive episodes.
Meperidine, a synthetic opioid, is used as an analgesic (by bolus administration) with sedation for short procedures and is less potent than fentanyl. Although chronic use of meperidine has risks, including seizures associated with the metabolite normeperidine and delirium, very brief courses and limited doses can be used safely. In fact, meperidine has been widely used as an analgesic in the sedation of patients undergoing outpatient-based, invasive diagnostic procedures without serious adverse effect [
22]. Meperidine has a fast onset of action, reaching peak effectiveness in 5–7 minutes, and has a duration of 2–4 hours when administered intravenously [
23]. In our study, 7–8 minutes elapsed between applying the monitor and performing the core puncture; therefore, delivering a meperidine bolus injection immediately after applying the monitor would provide peak effectiveness at the time of core puncture and in the postprocedural period. Peters et al. [
17] reported a mean visual analogue scale (VAS) value of 2.07 (1.5–2.65) for propofol sedation in a retrospective study, while Kang et al. [
18] reported a mean VAS value of 0.9 ± 1.1 for propofol with remifentanil sedation. These results were similar to the pain scores reported by the control group in our study. However, many studies have failed to determine a pain score or the incidence of pain in patients treated with propofol alone; therefore, we were unable to compare our results with these studies. Nevertheless, we noted a significant reduction of pain incidence in Group M. Specifically, all except two patients in Group M reported an NRS value of < 3, and meperidine was effective in decreasing moderate pain (NRS ≥ 4) incidence by 20%. Moreover, meperidine had a propofol- sparing effect without any periprocedural cardiorespiratory side effects. By virtue of the duration of meperidine, Group M showed lower SBP and DBP at 45 min and 60 min post-procedure; this could be a beneficial effect in elderly patients with hypertension.
Sedation for painful procedures compared to that of painless procedures is more difficult due to the possibility of movement. Few studies have attempted concomitant administration of sedatives and analgesics for TRUS-PBx [
18,
21]; the ones that have could not be compared with our study since most did not evaluate patient movement. In our study, despite meperidine pretreatment, the incidence of movement in Group M was 56.7%. However, Group M had 16.6% more patients who exhibited “no movement” and 13.3% fewer patients who exhibited “purposeful movement transiently interfering with the procedure” than those of Group C. Additionally, three patients in Group M only demonstrated minor movement during the last puncture of the biopsy, so they were not given additional i.v. propofol. Therefore, additional doses of propofol (two or more) were required in 40% of the patients in Group C, but in only 3.3% (one patient) in Group M. Even after an induction dose of i.v. propofol 2 mg/kg, the whole blood level decreases to 1.5 μg/ml, the level at which awakening usually occurs, approximately 8 min after injection due to rapid redistribution [
24]. To prevent drastic respiratory depression, we administered an initial bolus dose of propofol 1.5 mg/kg; this lower dose may have decreased the time to reach a blood propofol level for awakening to 5–7 minutes, and additional doses were thereby inevitable in 46.7% of Group M. Despite requiring additional propofol 5 minutes after the initial injection, even with meperidine pretreatment, the total propofol dose used was less than with continuous infusion [
16] and recovery was not delayed.
With respect to postprocedural side effects, meperidine decreased the incidence of tenesmus by approximately 30%. Clinically, tenesmus is a common complaint from patients undergoing TRUS-PBx, but it has received little attention. There are many sensory fibers in the portion of the anal canal distal to the dentate line, and the mechanical stretching of these fibers due to the introduction of the probe may cause discomfort, like tenesmus, to patients [
5]. Xu et al. [
25] reported that intramuscular meperidine administered 30 minutes before TRUS-PBx provided better analgesia than PPNB, especially during probe insertion, which corresponds well with our findings. Another study demonstrated that intravenous meperidine reduced pain during probe insertion more than periprostatic lidocaine infiltration, which could also support our findings [
26].
The anesthetic method used in this study can be applied to outpatient-based procedures with accompanying mild to moderate pain. With the concomitant use of meperidine, only a single dose of additional propofol, if any, is required; therefore, this method is easy and simple, and can reduce potential cardiorespiratory complications. Although deep sedation with concomitant analgesics requires hemodynamic monitoring, maintenance of a patent airway, preparation for positive ventilation, and surveillance by anesthesiologists, the elimination of all pain and discomfort is important for day care center patients and outpatients.
There were some limitations in our study. First, a group of patients not receiving any anesthesia was not included as a control; however, TRUS-PBx should not be performed without some type of anesthesia, as per international guidelines and recommendations. Second, we did not measure initial BP in the supine position; therefore, we could compare the intraprocedural BP with the initial BP (both recorded in the left lateral decubitus position), but could not compare the recovery period BP (recorded supine) with the initial BP. In addition, we did not measure BP immediately after administering the induction dose of propofol; we assumed that there would not be a marked decrease in BP since most patients experience high anxiety levels prior to a prostate biopsy and the procedure was performed immediately after loss of consciousness. Third, we did not evaluate the full recovery period, encompassing the end of the anesthesia to full awareness. However, all patients were sufficiently alert within 15 minutes of procedure completion to allow evaluation of their pain intensity NRS score. Lastly, our study did not include information concerning long-term complications, such as urinary retention, hematuria, hematospermia, fever, and use of analgesics after discharge.
In conclusion, meperidine pretreatment in association with propofol deep sedation can be an effective and safe analgesic method for reducing the pain and discomfort of patients undergoing TRUS-PBx.
Go to :








 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download