CASE REPORT
A 46-year-old man (173 cm, 67.5 kg) was scheduled to undergo video-assisted thoracoscopic wedge resection of the right lower lobe of the lung for removal of a hamartoma that had been incidentally discovered through a regular health screening. He had no specific medical history, and no abnormal findings were present in his preoperative laboratory data.
On arrival in the operating theater, his preanesthetic blood pressure (BP) was 110/70 mmHg, heart rate (HR) was 46 beats/min, respiratory rate was 18 breaths/min, and oxygen saturation as measured by pulse oximetry was 100%.
Anesthetic induction and maintenance were performed by target-controlled infusion of propofol–remifentanil and rocuronium. Immediately after induction of anesthesia, a 22-gauge catheter was inserted into the left radial artery for continuous arterial BP monitoring and intraoperative blood gas analysis. The operation was conducted under right lung collapse (left one-lung ventilation) via a 37-Fr double-lumen endotracheal tube, and the patient remained in the left lateral decubitus position throughout the surgery. Intraoperative arterial blood gas analysis showed a pH of 7.402, PaCO2 of 40.2 mmHg, PaO2 of 137.9 mmHg, HCO3− of 25.2 mEq/L, glucose level of 70 mg/dl (which was measured during one-lung ventilation), and fraction of inspired oxygen of 0.6. During the surgery, no adverse events or hemodynamic instabilities occurred except bleeding due to a tear in the lateral basal segmental vein of the right lung, which was immediately controlled by the surgeon without difficulty. In total, 800 ml normal saline and 450 ml plasma solution were administered. The total urinary output was 440 ml. The estimated blood loss volume was 150 ml as determined by the surgeon upon completion of surgery. The anesthesia and operation times were 235 min and 180 min, respectively.
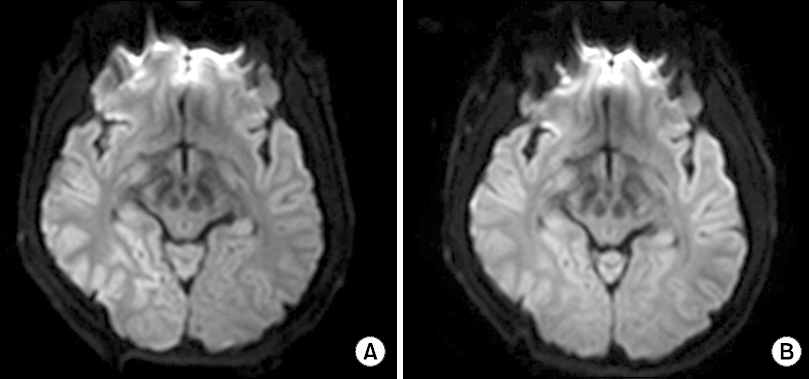
At the end of surgery, the patient was extubated after confirming response to verbal stimulation (“open your eyes”) and recovery of spontaneous breathing. The patient was moved to the postanesthesia care unit (PACU), and his initial vital signs were BP 127/82 mmHg, HR 86 beats/min, respiratory rate 18 breaths/min, and tympanic temperature 35.9°C. In the PACU, the patient’s vital signs remained stable and he was alert. He had no difficulty in communicating with others. Fifty minutes later, he was transferred to a general ward, where he developed gradual somnolence and lethargy and then slept for about 6 hours. After awakening, he complained of sudden and complete visual loss with headache and dizziness. At that time, his vital signs were BP 110/70 mmHg, HR 81 beats/min, respiratory rate 20 breaths/min, and temperature 36.7°C. He became drowsy and was supplied with oxygen (2 L/min) via a nasal prong. Examination by an ophthalmologist showed a normal pupillary light reflex (PLR) and no relative afferent pupillary defect. Neurologic examination by a neurologist revealed an intact ophthalmocephalic reflex, minimal left-sided facial palsy, and left-sided motor weakness. Brain computed tomography showed no remarkable findings. Magnetic resonance imaging (MRI) showed diffuse cortical high-signal intensity (
Fig. 1A). To examine the possibility of encephalitis or meningitis, a neurologist performed spinal tapping at the L3–L4 intervertebral space. The cerebrospinal fluid (CSF) pressure was 22 cmH
2O and its color was clear. Laboratory examination of the CSF also showed no abnormal findings, excluding an infectious cause. Blood testing showed mild elevation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels at 42 IU (reference range, 10–36 IU) and 40 IU (reference range, 7–38 IU), respectively, but all other parameters were unremarkable.
Fig. 1
Diffusion-weighted axial magnetic resonance images. (A) Initial imaging was taken on the day vision was lost and showed a hyperintense signal in the right temporo-occipital area. (B) After 13 days, the hyperintensity of the initial lesions had almost completely resolved.
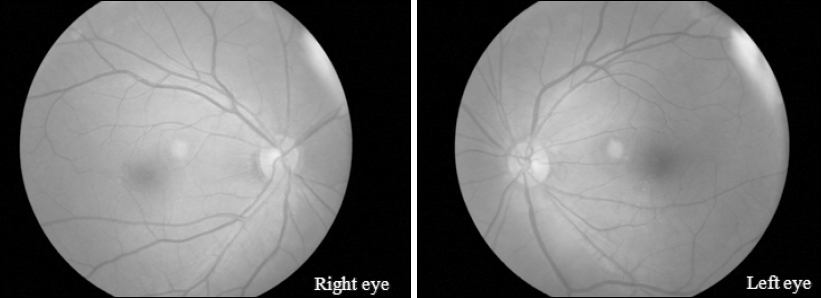
On postoperative day 1, the patient became drowsy and developed a fever (38.8°C) and headache. The fever was controlled by two 30-mg intravenous ketorolac injections, but the headache did not resolve. An electroencephalogram (EEG) was obtained to evaluate and differentiate any pathological brain conditions. The EEG findings as interpreted by a neurologist were suggestive of severe diffuse cerebral dysfunction that was worse in the right hemisphere, but no definite epileptiform discharge was identified. An ophthalmologist performed a fundoscopic examination to evaluate the cause of visual loss, but the findings were normal (
Fig. 2). The patient was transferred to the Department of Neurology and admitted to the intensive care unit (ICU). In the ICU, the patient was irritable, so he was restrained after obtaining informed consent from his brother. The neurologist prescribed oral aspirin at 100 mg/day because of the possibility of cerebral infarction.
Fig. 2
Fundoscopic findings on the day after onset of visual loss. Both images show a normal ocular fundus.
On postoperative day two, the patient became drowsy and confused, and continued to complain of visual loss, headache, and nausea. Laboratory examination revealed AST and ALT levels of 280 IU and 90 IU, respectively, and a creatine phosphokinase level of 24,200 IU (reference range, 0–170 IU). He was diagnosed with rhabdomyolysis caused by a muscle injury from his lack of cooperation in the ICU. Thus, the patient was treated with hydration and furosemide injections to maintain a urine output of 100 ml/h.
On postoperative day three, the patient became alert; his visual abnormality improved and he was able to read a book. However, although his visual loss resolved, his headache persisted. Follow-up brain MRI revealed a high signal intensity in the right hemispheric cortex (
Fig. 3). Based on his clinical manifestations and the results of neuroradiologic studies, the neurologist strongly suspected PRES. Hemodynamic changes from the day of surgery (onset day of visual loss) to postoperative day three (recovery day of visual loss) are presented in
Table 1.
Fig. 3
Magnetic resonance images at 3 days after onset of visual loss. Both (A) axial fluid-attenuated inversion recovery and (B) apparent diffusion coefficient show a high-intensity lesion (arrows) indicative of vasogenic cerebral edema.
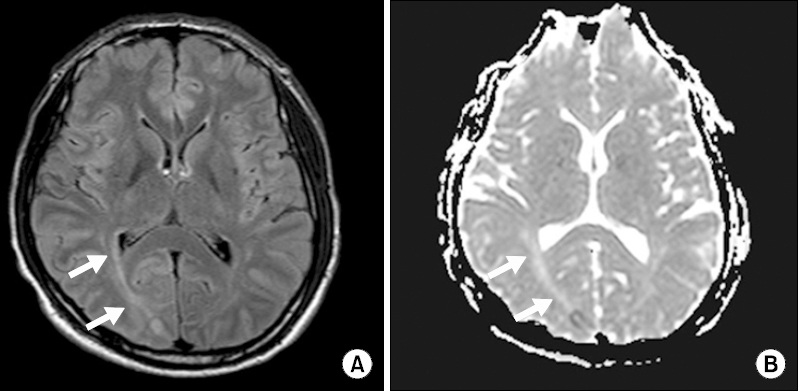
Table 1
|
Onset day of visual loss |
POD 1 |
POD 2 |
POD 3 |
|
|
Intraoperative |
In PACU |
In ward |
|
SBP (mmHg) |
95–138 |
111–132 |
110–120 |
100–116 |
90–130 |
100–124 |
|
DBP (mmHg) |
64–74 |
54–84 |
70–80 |
58–70 |
52–80 |
59–72 |
|
Heart rate (beats/min) |
46–96 |
85–99 |
78–97 |
62–98 |
56–105 |
51–97 |
Until 6 days after the operation, the patient complained of a mild sustained headache and was closely monitored for exacerbation of brain infarction or PRES in the ICU. Seven days after the operation, the patient was transferred to a general ward because he was no longer complaining of any neurologic symptoms and his AST/ALT and creatine phosphokinase levels had declined to 104/73 IU and 3,410 IU, respectively. On postoperative day 13, follow-up brain MRI showed resolution of the high signal intensity in the right cerebral hemisphere (
Fig. 1B). He was discharged from the hospital with no focal neurological deficits.
DISCUSSION
Postoperative visual loss usually results from ischemic optic neuropathy (ION) (59%), central retinal artery occlusion (CRAO) (24%), or cortical blindness (8%) after spinal or cardiac operations. Contributing factors include a prolonged operative time in the prone position, excessive blood loss, large-volume fluid resuscitation, and hypotension [
1]. None of these contributing factors was applicable to our patient.
ION can be categorized into anterior ION and posterior ION. Anterior ION can result from occlusion or relative hypoperfusion of the anterior optic nerve head by the posterior ciliary arteries, most frequently during cardiac surgery [
6], while posterior ION is caused by infarction of the intraorbital optic nerve, supplied by the pial blood vessels, usually during prone spinal surgery or radical neck dissection [
6]. CRAO results from a decreased blood supply to the entire retina; its most common cause is improper head positioning, which gives rise to external compression of the eye [
1]. Cortical blindness is generally associated with cerebral hypoperfusion in cases of prolonged hypotension, cerebral hypoxia, and procedures with high embolic loads such as angiography [
7,
8].
In contrast to the present case, on ophthalmologic examination, ION and CRAO patients have a relative afferent pupillary defect, absent PLR, or abnormal fundoscopic findings [
1]. Therefore, we were able to exclude CRAO and ION as differential diagnoses in our patient. We initially suspected cortical blindness, which usually presents with a normal PLR and fundus and is associated with stroke or embolism [
1]. However, resolution of the radiologic lesions implied vasogenic edema rather than brain infarction [
2,
3]. Considering the patient’s somnolence, dizziness, headache, altered mental status, nausea, abnormal neuroradiologic findings, vasogenic edema, and cortical visual loss, PRES was highly suspected. Additionally, reversible cortical blindness may occur as the presenting symptom of PRES [
2].
In previously reported cases, PRES occurred mostly in patients with underlying conditions such as hypertension, kidney disease, postpartum eclampsia, transplantation, immunosuppressant therapy, fluid retention, or intraoperative BP fluctuation [
4,
5]. Although the pathophysiology of PRES remains unclear and PRES can occur in patients who are normotensive, its pathophysiology is usually explained by cytotoxic or vasogenic theory; both are associated with an elevated BP surpassing the upper limit of cerebral autoregulation [
5,
9]. However, the cause of PRES was difficult to determine in the present case because the patient had no underlying conditions and his perioperative BP stayed within the normal range. Possible explanations for this are as follows. First, when the lateral basal segmental vein of the right lung was torn during the operation, an embolic event might have occurred and emboli could have entered the systemic circulation. Consequently, the cerebral autoregulation capability could have been damaged, causing cerebral vasodilatation and vasogenic cerebral edema [
3,
5]. Second, we may have missed a sudden and rapid elevation of BP in the PACU or general ward, where the BP was measured by noninvasive monitoring every 5 and 60 min, respectively. Third, our patient might have had a lower upper limit of autoregulation than other normal individuals.
In most cases, PRES begins with lethargy and somnolence, as in our patient [
3]. Subsequent neurologic abnormalities include encephalopathy (50–80%), seizures (60–75%), visual disturbances (33–60%), and headache (52–53%) [
4,
10,
11]. Mental function changes, dizziness, nausea, and vomiting may also develop [
10]. Visual abnormalities present as cortical blindness, hemianopia, blurred vision, and visual field deficits; however, the PLR and fundoscopy examination are always normal [
5].
Because lethargy, somnolence, dizziness, mild headache, nausea, and vomiting are common nonspecific symptoms in patients who have undergone anesthesia, anesthesiologists often overlook these symptoms. In some patients with PRES who underwent recent general or regional anesthesia, diagnostic procedures were performed after the severe persistent headache or seizure occurred [
12,
13]. As in other reported cases, although our patient showed somnolence and lethargy, these symptoms were regarded as residual anesthetic effects.
A diagnosis of PRES is based on clinical and neuroradiologic findings [
2,
11]. Among the neuroradiologic modalities, MRI is most helpful to diagnose PRES, as it can reveal vasogenic edema in the parieto-occipital regions of both cerebral hemispheres. The edema is commonly asymmetric, but almost always bilateral [
4,
11]. However, the location of the radiologic findings varies and can include unilateral lesions, gray matter/cortical lesions in anterior regions of the cortex, hemorrhage, or foci of permanent injury [
4].
In our patient, the vasogenic edema was not obvious in the left cerebral hemisphere; it was observed mainly in the right posterior temporal and parieto-occipital lobes. Fluid-attenuated inversion recovery (FLAIR) MRI is very sensitive for the detection of cerebral edema because it eliminates the confounding high signal from ventricular and subarachnoid CSF [
11,
14]. Diffusion-weighted imaging (DWI) and the apparent diffusion coefficient (ADC) can also be used to diagnose PRES. An increased FLAIR signal and bright ADC maps represent vasogenic edema [
15]. An increased DWI signal can be seen in cytotoxic edema, acute cerebral infarction, and areas of vasogenic edema with increased water content [
15]. In some cases of PRES, vasogenic edema can coexist with cytotoxic edema, which presents as dark areas on ADC maps and must be differentiated from acute cerebral infarction [
2]. In the present case, although neither the initial nor last follow-up brain MRI examination contained the FLAIR sequence, the second follow-up brain MRI examination showed high signal intensity on DWI, FLAIR, and ADC maps, indicating vasogenic edema. Nevertheless, the neurologist administered aspirin to prevent acute cerebral infarction. In our patient, despite the complete recovery of clinical symptoms, complete radiologic reversibility could not be identified because of the absence of a follow-up FLAIR sequence. However, evidence of radiological reversibility is not always necessary for a diagnosis of PRES [
11].
The key to management of PRES is strict control of BP using antihypertensive drugs such as nitroprusside, labetalol, and calcium channel blockers. Headache is difficult to control by analgesics but is relieved by treatment of hypertension [
9]. Unlike in cases of acute stroke or cerebral ischemia, hypertension in PRES should be treated aggressively to prevent progression to irreversible permanent cerebral infarction or visual loss [
15]. Other management techniques include withdrawal or dose reduction of immunosuppressants or cytotoxic drugs and administration of anticonvulsants in patients with seizures. Because our patient did not show an elevated BP or a definite epileptiform discharge focus on EEG, he received no antihypertensive or anticonvulsant drugs.
Generally, symptoms of PRES spontaneously resolve within 5.3 to 7.5 days with resolution of the radiological lesions [
4,
10]. Despite its name, however, PRES is not always reversible. Early diagnosis and prompt treatment are very important because PRES can progress to permanent brain damage, leukomalacia, or even death unless it is diagnosed and treated early [
5,
11,
15].
In conclusion, we experienced a case of PRES in a patient who underwent lung surgery in the lateral decubitus position and had no history of medical problems. Anesthesiologists should keep in mind the possibility of visual loss as a complication of anesthesia regardless of the type of surgery and the patient’s underlying disease. If the patient awakens from anesthesia and exhibits somnolence and lethargy followed by visual loss, the anesthesiologist should consider the possibility of PRES and promptly order neuroradiologic examination to ensure an early diagnosis and proper management.





