Abstract
The fibromyalgia syndrome (FMS) could be approached by various treatments modalities including education, aerobic exercise, cognitive behavioral therapy, tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, pregabalin, and so on. If other treatments fail, opioids including morphine should be considered. In this case report, we describe the case of a 44-year-old woman who was diagnosed with FMS three years ago, and suffered from severe intractable pain, side effects from other drugs, and opioid tolerance. Administration of morphine via an implantable drug delivery system resulted in significant improvement in the patient’s pain intensity, fibromyalgia impact questionnaire score, and sleep disturbance. Our case demonstrates that an implantable drug delivery system with morphine can be a potential treatment option for refractory fibromyalgia patients.
Go to : 
The fibromyalgia syndrome (FMS) is a chronic pain disease characterized by widespread pain, fatigue, sleep disturbance, depressive moods, and cognitive impairment. According to the modified 2010 American College of Rheumatology criteria, the prevalence of fibromyalgia is estimated to be 5.4% in the US and 1.7% in Korea [1,2]. The pathophysiology of FMS is not fully understood. Psychological or biological stress, decreased descending analgesic activity, increased activity of ascending pain transmission pathways, genetic factors, and central sensitization secondary to persistent peripheral noxious input are considered to be characteristics of FMS pathophysiology [3].
Most of FMS could be managed by various nonpharmacological and pharmacological therapies including pregabalin and selective noradrenaline reuptake inhibitors. However, a few patients suffer from intractable severe pain in spite of various efforts. In these intractable FMS, opioids can be considered carefully [3].
Continuous intrathecal analgesic administration such as that using an implantable drug delivery system (IDDS) can be considered when chronic pain recurs after an initial response to strong opioids or interventional pain treatments are not successful [4]. We expected that intrathecal opioid administration is more effective to reduce central sensitized pain and less systemic side effect than systemic opioid administration in FMS. However, there is no literature about an IDDS with morphine in FMS.
We report the case of an IDDS in an FMS patient who suffered from side effects of other FMS treatments and opioid tolerance.
Go to : 
A 44-year-old woman was diagnosed with FMS three years ago. She had a score of 8 on the numeric rating scale for pain (NRS; where 0 indicates no pain and 10, the worst pain imaginable). The fibromyalgia impact questionnaire (FIQ) score was 79 points. Moreover, 13 out of 18 tender points were painful, and the patient complained of widespread pain, especially in the upper back; sleep disturbance; fatigue; and anxiety. The patient met the clinical criteria for FMS based on the American College of Rheumatology 2010 Criteria (widespread pain index was 10 and symptom severity index was 8) [2]. She had no other medical problems. In the early stage of the disease, she had been treated with pregabalin and milnacipran. However, side effects, such as serious dizziness and drowsiness, with 75 mg/day pregabalin and severe nausea with 12.5 mg/day milnacipran had forced her to abandon these treatments. We made several attempts to titrate the dosage of the first-line drugs, such as pregabalin and milnacipran, to achieve the desired effect, but she was not tolerant to the side effects. She received exercise therapy, a high-dose ketamine infusion, repeated transcranial magnetic stimulation, and psychotherapy. However, these therapies had no effect. She began to receive opioids (12.5 μg/h transdermal fentanyl patch) two years after the diagnosis, but this was not used in combination with pregabalin and milnacipran because of the side effects. Only opioids were able to reduce her pain. The patient received 800 μg transmucosal fentanyl citrate, a 50 μg/h transdermal fentanyl patch, 24 mg/day hydromorphone and 0.25 mg/day clonazepam. The equivalent morphine dose was 270 mg/day orally. At that time, her NRS score was 4–5 out of 10. However, the effectiveness of opioids gradually decreased because of opioid tolerance. The pain intensity increased from 4 to 8 out of 10 on the NRS. Sleep disturbance worsened. Although the opioid dose was increased to an equivalent morphine dose of 305 mg/day, the pain relief was insufficient and temporary. Since the patient had no opioid addiction and second gain according to a psychiatric interview, it was decided to introduce an IDDS.
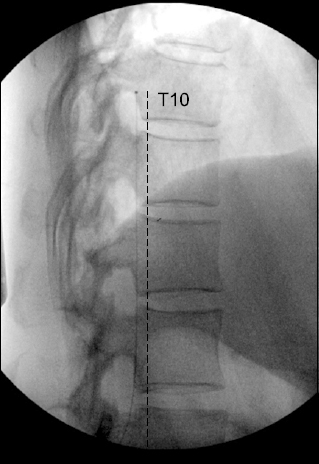
An epidural morphine infusion was carried out to test the intrathecal morphine pump implant. The epidural catheter tip was positioned at the T10 level; the starting morphine dose was 10 mg/day, which was increased to 20 mg/day for five days. The pain intensity decreased from 8 to 4 out of 10 on the NRS, and side effects including constipation, nausea, and respiratory depression were not seen during the trial period. We implanted an intrathecal drug delivery system (Synchromed® II drug pump; Medtronic, USA) with the catheter tip positioned at the T10 level (Fig. 1). An intrathecal morphine sulfate infusion was started at the rate of 1.0 mg/day. Over a period of three months, the morphine sulfate infusion dosage was increased for titration, until it reached 3.0 mg/day, and the patient’s NRS score became 3 or 4. Sleep disturbance also decreased. The FIQ score decreased from 79 to 47. Breakthrough pain (mainly in the upper back), which occurred on average twice a day, was controlled with 600 μg transmucosal fentanyl. Medication for background pain was unnecessary. Over one year, IDDS with morphine maintained without adverse event. Morphine dose escalation was done, till it reached to 4.0 mg/day. During the last two months, the daily opioid dose remained stable. Her pain intensity maintained below moderate pain. The patient was able to work and perform mild exercises such as stretching and walking for one hour, and she was satisfied with the intrathecal morphine pump.
Go to : 
The goal of FMS treatment is usually symptom improvement and functional maintenance. Multidisciplinary treatment combining pharmacological and non-pharmacological approaches is recommended by experts. Duloxetine, milnacipran, and pregabalin have shown efficacy in several randomized, double-blind clinical trials; so, these three drugs are used as first-line pharmacological therapy in FMS [5]. The discontinuation rate for these three drugs due to adverse events ranged from 11.4% to 27.2% (duloxetine), from 13.7% to 28.2% (milnacipran), and from 7.5% to 32.6% (pregabalin) [5]. When first-line drugs are not suitable, nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, selective serotonin reuptake inhibitors, skeletal muscle relaxants, and opioid are considered [3]. In our case, we tried intravenous ketamine infusion and repeated transcranial magnetic stimulation (rTMS) after the treatment with the first-line drugs failed. Ketamine, N-methyl-D-aspartate (NMDA) receptor antagonist can reduce both thermal and mechanical wind-up in patients with FMS, although it does not have long-term analgesic effect [3]. Low-intensity rTMS may also be an effective treatment option for patients with FMS for improving the pain threshold [6].
Opioid treatment in FMS is indicated for those who have severe pain, efficacy failures, and cannot tolerate any other therapy [3,5]. Although the use of opioids in FMS remains controversial, they are commonly used clinically. In a US-based prospective study of FMS treatment patterns, opioid and tramadol prescribed 24.2% and 15.3%, respectively [7].
There is limited literature about epidural or intrathecal opioid administration in FMS. In a small study (with 9 patients), an epidural opioid injection reduced resting pain, the number of tender points [8]. It is well known that there is a three-fold elevation of substance P (SP), which is an excitatory neurotransmitter released at pre-and postsynaptic dorsal horn neurons, in the cerebrospinal fluid of FMS patients [9]. The release of SP enables the removal of the magnesium block from the NMDA receptor, which allows the wind-up phenomenon in the spinal cord [3]. An experimental animal study showed that an intrathecal opioid infusion was more effective for suppressing the release of SP than a subcutaneous opioid injection [10]. An intrathecal opioid infusion also acts on the dorsal horn of the spinal cord by presynaptic and postsynaptic interaction; an opioid inhibits the release of SP and calcitonin gene-related peptide which is related to presynaptic calcium influx and activate the postsynaptic opioid receptors, which is finally leading to less postsynaptic second-order neuron responsiveness [4].
Continuous intrathecal opioid administration was indicated for those with opioid responsiveness but failure of systemic opioid therapy due to high dose requirements or intolerable side effects [4]. Long-term intrathecal opioid treatment for chronic non-malignant pain reduced pain intensity by approximately 60%, and improved the activity, mood, and quality of life [11]. The problems associated with IDDS are infection, catheter-related problems, pump malfunction, and drug-related problems. Among them, granuloma formation is a serious complication [11]. High concentrations (20 mg/ml), high daily doses (above 15 mg/day), and low flow rates for intrathecal morphine or hydromorphone infusions increase the risk of developing catheter-tip granulomas [12]. We expected that an IDDS in FMS would inhibit the central sensitization of pain [10], and would have fewer side effects than systemic opioid treatment [12]. In systemic review, the incidence of psychological dependence was low rate in who has no history of abuse behavior [13]. The mean opioid dose steadily increased during the initial 6 to 24 months; however, this has been relatively stable over the past 6 years in patients with chronic non-cancer pain [14].
The distribution of intrathecal morphine sulfate is known to be associated with the catheter tip position. Moulin et al. [15] demonstrated that the ratio of the lumbar to cisternal (L/C) concentration of morphine sulfate with the catheter tip at L2 was higher than that at T10. Because of these pharmacokinetic characteristics of morphine sulfate, the level of the catheter tip, pain lesion, and occurrence of complication due to morphine sulfate must be considered when an IDDS is performed. In this case, the main lesion causing the pain was in the upper back, and therefore, we decided that the lower thoracic level was the appropriate position for the catheter tip.
In our case, we introduced an IDDS with morphine sulfate in a FMS patient who suffered from intractable pain and was resistant to other therapies. This case showed that an IDDS with morphine reduced pain and the FIQ score, and improved sleep disturbance.
In conclusion, FMS patients have chronic pain, which is difficult to treat. If other treatments fail or are not tolerated, an IDDS with morphine should be considered for those who had a successful intrathecal or epidural opioid trial. This is can be a potential treatment option for patients with refractory fibromyalgia.
Go to : 
REFERENCES
1. Kim C, Kim H, Kim J. Prevalence of chronic widespread pain and fibromyalgia syndrome: a Korean hospital-based study. Rheumatol Int. 2012; 32:3435–42. DOI: 10.1007/s00296-011-2195-1. PMID: 22057141.
2. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken). 2010; 62:600–10. DOI: 10.1002/acr.20140. PMID: 20461783.
3. Jay GW, Barkin RL. Fibromyalgia. Dis Mon. 2015; 61:66–111. DOI: 10.1016/j.disamonth.2015.01.002. PMID: 25769552.
4. Ver Donck A, Vranken JH, Puylaert M, Hayek S, Mekhail N, Van Zundert J. Intrathecal drug administration in chronic pain syndromes. Pain Pract. 2014; 14:461–76. PMID: 24118774.
5. Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother. 2015; 16:1347–68. DOI: 10.1517/14656566.2015.1047343. PMID: 26001183.
6. Maestú C, Blanco M, Nevado A, Romero J, Rodríguez-Rubio P, Galindo J, et al. Reduction of pain thresholds in fibromyalgia after very low-intensity magnetic stimulation: a double-blinded, randomized placebo-controlled clinical trial. Pain Res Manag. 2013; 18:e101–6. DOI: 10.1155/2013/270183. PMID: 24308025. PMCID: PMC3917800.
7. Robinson RL, Kroenke K, Mease P, Williams DA, Chen Y, D’Souza D, et al. Burden of illness and treatment patterns for patients with fibromyalgia. Pain Med. 2012; 13:1366–76. DOI: 10.1111/j.1526-4637.2012.01475.x. PMID: 22958298.
8. Bengtsson M, Bengtsson A, Jorfeldt L. Diagnostic epidural opioid blockade in primary fibromyalgia at rest and during exercise. Pain. 1989; 39:171–80. DOI: 10.1016/0304-3959(89)90004-3.
9. Russell IJ, Orr MD, Littman B, Vipraio GA, Alboukrek D, Michalek JE, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994; 37:1593–601. DOI: 10.1002/art.1780371106. PMID: 7526868.
10. Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, et al. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005; 25:3651–60. DOI: 10.1523/JNEUROSCI.0252-05.2005. PMID: 15814796.
11. Falco FJ, Patel VB, Hayek SM, Deer TR, Geffert S, Zhu J, et al. Intrathecal infusion systems for long-term management of chronic non-cancer pain: an update of assessment of evidence. Pain Physician. 2013; 16(2 Suppl):SE185–216. PMID: 23615891.
12. Rhee SM, Choi EJ, Lee PB, Nahm FS. Catheter obstruction of intrathecal drug administration system -a case report-. Korean J Pain. 2012; 25:47–51. DOI: 10.3344/kjp.2012.25.1.47. PMID: 22259717. PMCID: PMC3259138.
13. Duarte RV, Raphael JH, Sparkes E, Southall JL, LeMarchand K, Ashford RL. Long-term intrathecal drug administration for chronic nonmalignant pain. J Neurosurg Anesthesiol. 2012; 24:63–70. DOI: 10.1097/ANA.0b013e31822ff779. PMID: 21904220.
14. Duarte RV, Raphael JH, Haque MS, Southall JL, Ashford RL. A predictive model for intrathecal opioid dose escalation for chronic non-cancer pain. Pain Physician. 2012; 15:363–9. PMID: 22996848.
15. Moulin DE, Inturrisi CE, Foley KM. Cerebrospinal fluid pharmacokinetics of intrathecal morphine sulfate and D-Ala2-D-Leu5-en kephalin. Ann Neurol. 1986; 20:218–22. DOI: 10.1002/ana.410200207. PMID: 3530119.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download