This article has been
cited by other articles in ScienceCentral.
Abstract
Background:
In emergency condition, failure in securing airway is a common and serious reason of pediatric death. Rapid intubation is required to minimize physiologic complication in children due to airway failure. Rapid loss of consciousness and rapid onset of neuromuscular blocking agent are necessary for the rapid sequence intubation. In this study, we compared the effects of thiopental sodium, ketamine, and propofol (drugs commonly used to induce anesthesia in children) on the onset time of rocuronium. We also compared the effects of these anesthesia induction drugs on intubation condition and their duration of action.
Methods:
A total of 89 patients undergoing various elective surgeries were enrolled and allocated to the following three groups according to the anesthesia induction drug: 1) Group T, thiopental sodium; 2) Group P, propofol; and 3) Group K, ketamine. After loss of consciousness, neuromuscular monitoring was performed and rocurunium 0.6 mg/kg was administered. Onset time and duration of action of rocuronium were measured. Intubation condition was recorded with a tracheal intubation scoring system. Hemodynamic changes were observed before induction until 5 min after endotracheal intubation.
Results:
The onset time of rocuronium in group K (39.9 s) was significantly faster than that in group T (61.7 s) or group P (50.7 s). There was no significant difference in duration of action of rocuronium or intubation condition among the three groups.
Conclusions:
Ketamine can decrease the onset time of rocuronium significantly compared to thiopental sodium or propofol.
Keywords: Ketamine, Propofol, Rocuronium, Thiopental sodium
INTRODUCTION
Airway management is the first priority in emergency care of pediatric patients. Endotracheal intubation is a definite procedure in optimal management of the airway [
1]. Rapid sequence intubation (RSI) is commonly used in emergency situation to facilitate the securement of an airway. It has two basic technical components: tracheal intubation with direct laryngoscopy and induction of general anesthesia [
1]. Simultaneous administration of intravenous induction agents and neuromuscular blocking agent (NMBA) is often required for the induction of general anesthesia during RSI to facilitate emergent endotracheal intubation.
NMBAs are integral to successful RSI. They can reduce the risk of complications [
2]. Although succinylcholine remains the preferred NMBA for RSI, there are arguments on whether it should be used due to its side effects such as cardiac arrest and malignant hyperthermia that have occurred in relatively predictable circumstances [
3]. Recently, rocuronium is widely used for RSI because of its rapid onset [
3]. However, the onset time of rocuronium can change based on changes of cardiac output [
4]. Its prolonged duration of action compared to succinylcholine can cause problem during emergency situation [
3].
Thiopental sodium, ketamine, and propofol are frequently used to induce anesthesia in children. These intravenous induction agents can produce hemodynamic changes and influence cardiac output [
5]. Therefore, the onset time of rocuronium can change according to the induction drugs [
6]. The objective of this study was to compare the effects of thiopental sodium, ketamine, and propofol on the onset time of rocuronium. We also compared their effects on intubation condition and their duration of action.
MATERIALS AND METHODS
The study was conducted after obtaining approval from the Institutional Review Board. A total of 90 patients who were scheduled for elective surgery, aged 3 to 10 years, and American Society of Anesthesiologists (ASA) class I or II were enrolled. Patients who had difficult airway, neuromuscular diseases, allergies to anesthetics and NMBA, and those who had neurologic deficit were excluded. Informed consent was obtained from all patients’ parents after full explanation of the aim and method of the study. Patients were allocated to three groups according to the anesthetic induction drug to be used through a computerized randomization. Patients in group T received thiopental sodium 5 mg/kg for the induction of anesthesia. Those in group P received propofol 2.5 mg/kg for the induction of anesthesia. Patients in group K received ketamine 2.0 mg/kg for the induction of anesthesia [
7].
No drug has been used before the induction of anesthesia. Parents of patients were allowed to stay in the operating room until the initiation of anesthesia induction. After arrival at the operating room, standard monitoring devices such as electrocardiogram, pulse oximetry, and non-invasive blood pressure were attached to patients. Neuromuscular monitoring was performed using Pediatric Acceleromyography Seonsor (Datex-Ohmeda Inc., Finland). Two pediatric surface electrodes were attached onto the ulnar side of the wrist with a spacing of 3 cm. Pediatric acceleromyography was fixed on the 1st finger and the thumb. After that, preoxygenation was applied for 3 min through a facemask before the induction of anesthesia.
Induction drug was injected to induce unconsciousness. Ventilation was started with a face mask. After administration of the induction agent, no other drugs except a single dose of rocuronium was administered until the end of endotracheal intubation. The forearm and hand were immobilized with bandage to prevent motion artifacts. However, fingers were allowed to move freely.
At one minute after the end of the administration of induction drug and after confirming that the sensors were fixed firmly onto the 1st finger and thumb, the neuromuscular module performed an automatic search for the optimal stimulus current to achieve the maximum response of the adductor pollicis muscle. The corresponding electromyographic amplitudes were measured and displayed on an anesthetic monitoring system (Anesthetic Monitoring System S/5™, Datex-Ohmeda Inc., Finland). Neuromuscular function was assessed with a 0.1 Hz single twitch stimulation to assess the onset time and Train-of-four (TOF) stimuli to determine the duration of action according to the clinical research practice guidelines of neuromuscular blocking agents [
8]. A time interval of 10 seconds was used between stimulations. After measuring baseline value of single twitch, patients received a single dose of rocuronium 0.6 mg/kg within 5 seconds. After that, no more rocuronium was administered until the end of the surgery. The time from the end of rocuronium injection to achieving 95% suppression of single twitch was measured as an onset time. When the single twitch disappeared, endotracheal intubation was done. The intubation condition was measured with the intubation scoring system (ISS) according to Viby-Mogensen et al. [
9] (
Table 1). After endotracheal intubation was done, anesthesia was maintained with 2–3 vol% sevoflurane and 50% O
2-N
2O mixture. Sevoflurane was adjusted to maintain mean arterial pressure (MAP) within 20% of baseline values. When hemodynamic values could not be controlled by adjusting anesthetics, inotropics or vasodilator was used and patients were excluded from the study. After that, TOF was measured. Duration of action was defined as the time from the end of rocuronium injection until T1 of the TOF that had recovered to 25% of the control T1 value.
Table 1
Intubation Scoring System according to Viby-Mogensen et al. [
9]
|
Score |
Vocal cord |
Jaw relaxation |
Coughing or bucking |
|
3 |
Fully abducted |
Fully relaxed |
Nil |
|
2 |
Slightly abducted |
Slightly stiff |
Slight |
|
1 |
Partially abducted |
Stiff |
Moderate |
|
0 |
Closed |
Impossible to open |
Severe |
MAP and heart rate (HR) were measured with a time sequence: T1, baseline before induction; T2, 1 min after injection of anesthetics for induction; T3, just before endotracheal intubation; T4, 1 min after endotracheal intubation; T5, 2 mins after endotracheal intubation; T6, 3 mins after endotracheal intubation; T7, 4 mins after endotracheal intubation; T8: 5 mins after endotracheal intubation.
Sample size was calculated using “G*Power3” free software (available at:
http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3). Sample size needed for the onset time was calculated at effect size of 0.4 which was estimated using the standardized effect size of Cohen [
10]. Using α = 0.05 with a power of 80%, the total sample size was calculated to be 66 for the 3 groups. Considering a drop-out rate of approximately 30%, 30 patients were allocated to each group in this study.
Statistical analysis was performed using SPSS 12.0 (SPSS, USA). Data are expressed as means ± SD. ASA class and gender were compared among the three groups using Chi-Square test. Age, weight, height, onset time, duration of action, and ISS were analyzed by one-way analysis of variance (ANOVA). Hemodynamic changes were analyzed by using repeat measure ANOVA. When between-group differences were observed, Mann-Whitney U test was used to analyze the difference between the pairs. Post hoc tests were done with Turkey’s HSD. Statistical significance was considered when P value was less than 0.05.
RESULTS
A total of 90 patients were enrolled in this study and 89 patients were assessed in our final analysis. One patient in group K was excluded due to refusal to participate after surgery. There was no significant difference in demographic characteristics (
Table 2) among the three groups.
Table 2
Demographic Characteristics of Patients Used in the Three Groups
|
Group T |
Group P |
Group K |
|
(n = 30) |
(n = 30) |
(n = 29) |
|
Age (yr) |
7.0 ± 2.1 |
5.8 ± 1.9 |
6.7 ± 2.6 |
|
Gender (M/F) |
17/13 |
20/10 |
13/16 |
|
Weight (kg) |
27.6 ± 9.9 |
25.6 ± 9.9 |
25.5 ± 8.9 |
|
Height (cm) |
124.1 ± 14.4 |
118.1 ± 14.2 |
121.3 ± 15.4 |
|
ASA class (I/II) |
30/0 |
29/1 |
28/1 |
The onset time of rocuronium in group K was significantly faster than that in group T or group P (39.9 s vs. 61.7 or 50.7 s, P = 0.001,
Table 3). There was no significant difference in intubation condition or the duration of action of rocuronium among the three groups (intubation condition, P = 0.949; duration of action, P = 0.646,
Table 3).
Table 3
Onset Time, Duration of Action, and Intubation Condition
|
Group T |
Group P |
Group K |
|
(n = 30) |
(n = 30) |
(n = 29) |
|
Onset time (s) |
61.7 ± 19.5 |
50.7 ± 14.0 |
39.9 ± 11.8*,† |
|
Duration of action (min) |
30.8 ± 8.8 |
30.5 ± 7.7 |
32.3 ± 7.4 |
|
Intubation Score by ISS |
8.9 ± 0.6 |
8.8 ± 0.5 |
8.8 ± 0.4 |
At 1 min after injection of anesthetics for induction (T2), MAP was significantly increased in group K compared to that in group T or group P (both P < 0.001,
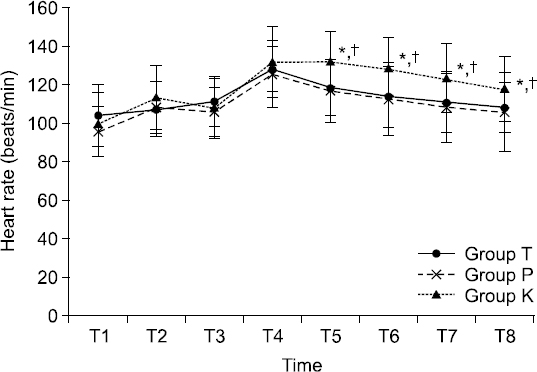
Fig. 1). HR was also significantly increased at 2 min after endotracheal intubation (T5) in group K compared to that in group T or group P (both P < 0.001,
Fig. 2).
Fig. 1
Values are mean ± SD. Sequential changes of mean arterial pressure (MAP). MAP was higher in group K than that in group P or group T after the injection of anesthetics. Group T: thiopental sodium, Groups P: propofol, Group K: ketamine. T1: baseline before induction, T2: 1 min after injection of anesthetics for induction, T3: just before endotracheal intubation, T4: 1 min after endotracheal intubation, T5: 2 mins after endotracheal intubation, T6: 3 mins after endotracheal intubation, T7: 4 mins after endotracheal intubation, T8: 5 mins after endotracheal intubation. *P < 0.05 compared to group T. †P < 0.05 compared to group P.
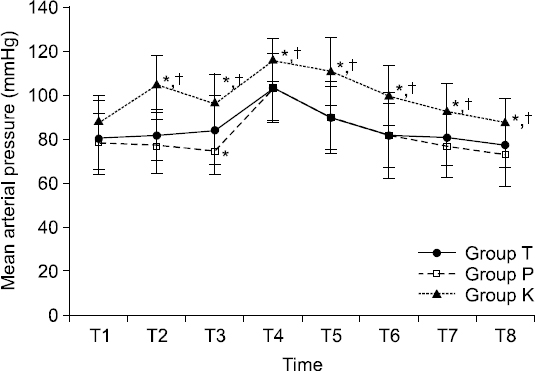
Fig. 2
Values are mean ± SD. Sequential changes of heart rate (HR). HR was higher in group K than that in group P or group T at 2 min after endotracheal intubation. Group T: thiopental sodium, Groups P: propofol, Group K: ketamine. T1: baseline before induction, T2: 1 min after injection of anesthetics for induction, T3: just before endotracheal intubation, T4: 1 min after endotracheal intubation, T5: 2 mins after endotracheal intubation, T6: 3 mins after endotracheal intubation, T7: 4 mins after endotracheal intubation, T8: 5 mins after endotracheal intubation. *P < 0.05 compared to group T. †P < 0.05 compared to group P.
DISCUSSION
In the current study, we compared the effects of thiopental sodium, ketamine, and propofol on rocuronium onset time and the duration of action of rocuronium. We hypothesized that changes produced by intravenous induction agents could affect the onset time of rocuronium and its duration of action. We expected faster onset of rocuronium by ketamine because of its ability of increasing cardiac output by increasing blood pressure and HR [
11]. Our results revealed that ketamine significantly shortened the onset time of rocuronium than thiopental sodium or propofol. However, there was no significant differences in intubation condition or the duration of action of rocuronium among the three groups.
Although the effect of cardiovascular factors on the onset time of NMBA has not been completely defined, it is partly determined by the time that the drug reaches the neuromuscular junction, cardiac output, and blood flow of muscle [
12]. A previous study has shown a close relation between the circulation time and the onset time of succinylcholine [
13]. Munoz et al. [
14] have reported that a single dose of ephedrine administered during induction can reduce the onset time of rocuronium. They have suggested that ephedrine might have increased cardiac output and muscle blood flow [
14]. Gill and Scott [
15] have also reported that the onset time of vecuronium is shorter when etomidate is used for induction compared to thiopental sodium because of the lesser hemodynamic depression of etomidate than thiopental sodium. Thus, we compared the onset time of rocuronium in this study according to the induction drug used.
When cardiovascular effects are compared between propofol and thiopental sodium, MAP has been found to be decreased greater after the administration of propofol than that after the administration of thiopental sodium [
16]. However, the reduction in cardiac index is not significantly different between propofol and thiopental sodium groups [
16]. In the current study, reduction of MAP was greater after the administration of propofol compared to that after the administration of thiopental sodium, although the different was not statistically significant. The reduction of MAP showed significance between thiopental sodium and propofol only when after intubation.
It has been reported that less pediatric patients have experienced significant MAP decreases (> 20% compared to baseline) during induction when ketamine, but not propofol, is used [
17]. In this study, MAP was increased after the injection of ketamine. The MAP levels in patients who were administered with ketamine were significantly higher than those who were administered with thiopental sodium or propofol. However, there were no significant changes among the groups in the HR until 1 min after endotracheal intubation.
It is well known that ketamine can override its negative inotropic effect and tend to cause an increase in blood pressure and HR because of its sympathomimetic properties [
11]. However, the mechanism involved in its cardiovascular effects remains unclear. Its effect can vary depending on patient condition and its dosage used [
18,
19]. It has been reported that ketamine does not alter HR or cardiac output or facilitate the onset of rocuronium [
20,
21]. After the administration of ketamine in pediatric patients with chronic pulmonary hypertension who have catecholamine depletion or are taking beta-blockers, there is no change in HR or cardiac output [
20]. Kim et al. [
21] have also reported that ketamine does not alter MAP or HR or accelerate rocuronium onset time compared to controls. The discrepancy in these results among various studies might be due to differences in study protocols. In the current study, we only enrolled pediatric patients with ASA class I–II. They had little possibility of having pulmonary hypertension. In addition, we only used ketamine at induction dose (2 mg/kg) in group K. In contrast, Kim et al. [
21] used ketamine as priming drug at a lower dosage (0.5 mg/kg). After that, propofol was used again for the induction of anesthesia. Such differences might have resulted in differences in MAP and cardiac output.
Interestingly, ketamine only increased MAP, not HR after its administration in the current study. Sympathomimetic properties of ketamine might be due to increased sympathetic nervous system activity [
22]. It has been suggested that norepinephrine release is enhanced when baroreceptor reflex activity is depressed [
22]. Moreover, ketamine can increase the levels of circulating catecholamine by reuptake inhibition not only in the central nervous system and heart, but also in the peripheral nervous system [
22]. Therefore, the increase of MBP secondary to the increase of systemic and peripheral vascular resistance with the induction of ketamine might have abolished its negative inotropic effects and baroreceptor reflex, leading to increased MBP without increasing HR. Further study is merited to confirm this hypothesis.
According to these hemodynamic results, a difference in cardiac output among groups is suspected. These results suggest that the increase of cardiac output in these patients who were administered with ketamine might have increased muscle blood flow. This might be the reason why the onset time of rocuronium is shortened after the use of ketamine compared to that after the use of thiopental sodium or propofol.
The current study had some limitations. First, we did not measure cardiac output or muscle blood flow directly. We only speculated the differences of hemodynamic variables after the use of ketamine. We hypothesized the mechanism involved in reduction of the onset time of rocuronium after the use of ketamine. Therefore, further evaluation with direct measurement of cardiac output is needed. Increased MAP during induction of anesthesia is another limitation of the current study. Sevoflurane was not used until the end of endotracheal intubation to prohibit its influence on neuromuscular blockage. Thus, light anesthesia could be the reason of the increase of MAP until 5 min after endotracheal intubation. However, sevoflurane was used after the endotracheal intubation. Moreover, MAP was maintained within 20% of its baseline value until the end of surgery by adjusting the volatile anesthetic agents.
In conclusion, we found that the use of ketamine as anesthesia induction agent could decrease the onset time of rocuronium than using thiopental sodium or propofol as anesthesia induction agent.






 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download