Allergic rhinitis (AR) is the most common immunological disease in people of all ages, and its prevalence is increasing globally [1]. Despite proper management, some AR patients experience persistent and severe symptoms [2]. Therefore, efforts to investigate new therapeutic modalities and drug combinations are underway. AR mouse models have been broadly used for various experiments ever since a Japanese research group introduced the ovalbumin (OVA)-induced model of AR [3]. Sneezing and rubbing counts in the mouse model of AR, which are assessed on the last day of the intranasal challenge by blinded observers, have been used as fundamental parameters for the objective measurement of symptom severity. However, the methods and time intervals used when measuring sneezing and rubbing counts have varied among researchers. Furthermore, it has not yet been clarified how well sneezing and rubbing counts reflect inflammatory manifestations in the AR mouse model. We sought to investigate whether sneezing and rubbing counts were correlated with other allergic parameters in the AR mouse model and to identify a more precise method of obtaining sneezing and rubbing counts as indicators of type 2 inflammation.
Female BALB/c mice were used to establish the AR mouse model, as previously described [4]. The AR model was established with OVA and alum sensitization, and the negative control group was challenged with phosphate-buffered saline. The negative control and positive control groups were recruited for analysis from different experiments. The Institutional Animal Care and Use Committee at the Clinical Research Institute of Dankook University Hospital approved all experiments. Before sacrifice, sneezing and rubbing counts were measured on day 28 during 0–20 minutes after the last OVA challenge at 5-minute intervals by two blinded observers (JHK and EHK). The scores were measured every 5 minutes, and the sum of the scores was analyzed. We sacrificed the mice 24 hours after the last OVA challenge and we harvested the nasal mucosa tissues from one side of the head using small curettes and micro-forceps under microscopic visualization. The other side of the head was fixed in 4% paraformaldehyde for a histologic evaluation of eosinophilic infiltration. We measured serum total immunoglobulin E (IgE), OVA-specific IgE, IgG1, and IgG2a and systemic cytokine levels of interleukin (IL)-4, IL-5, IL-6, IL-17A, and interferon gamma (IFN-γ) using splenic single-cell suspensions, as described in more detail in our previous article [5]. The levels of mRNA expression in the nasal mucosa were evaluated using real-time reverse transcription-polymerase chain reaction (RT-PCR).
In total, 142 mice were analyzed. The average sneezing and rubbing counts in the negative control (n=73) and positive control (n=69) groups were 27.8±16.8 and 116.9±67.2, respectively (P<0.001) (Fig. 1A). The symptom score of the first 5-minute interval was higher than that of the following intervals, and the 5-minute interval symptom scores gradually decreased over time in both groups (Fig. 1B). Eosinophilic infiltration in the nasal tissue was higher in the positive control group than in the negative control group (Fig. 1C). Type 2 inflammatory markers showed positive correlations with sneezing and rubbing counts (P<0.001), and the negative and positive control groups exhibited distinct patterns in the levels of inflammatory mediators (Fig. 2A). All parameters, except nasal IL-6 and IFN-γ mRNA levels, were significantly overexpressed in the positive control group compared with the negative control group (P<0.05).
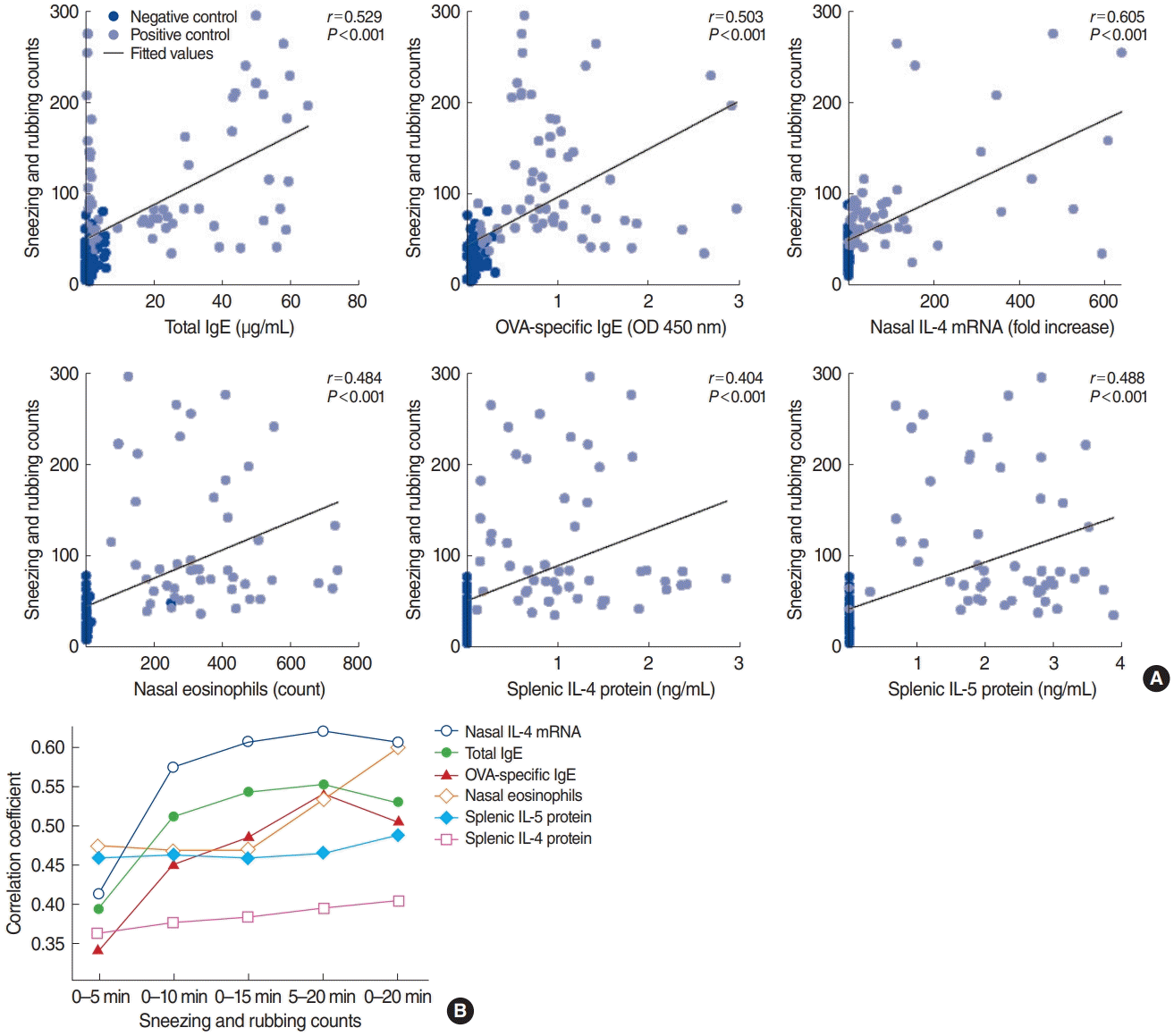
The symptom scores recorded for 0–20 minutes showed stronger correlations with type 2 allergic parameters, such as serum OVA-specific IgG2a (r=0.501, P<0.001), splenic IL-4 protein levels (r=0.404, P<0.001), splenic IL-5 protein levels (r=0.488, P<0.001), and eosinophilic infiltration (r=0.600, P<0.001), than were found for the sneezing and rubbing counts for 0–10 minutes, 0–15 minutes, and 5–20 minutes (Fig. 2B, Supplementary Table 1). The levels of the cytokines IL-6, IL-17A, and IFN-γ—either for enzyme-linked immunosorbent assay results from splenic cells or for RT-PCR results of the nasal tissues—showed no significant correlations with symptom scores. Overall, type 2 immune response mediators were closely correlated with the symptom score, and the correlation coefficient of nasal IL-4 mRNA levels was the highest among the type 2 inflammation markers.
In the present study, we evaluated the correlations between sneezing and rubbing counts and inflammatory markers for the first time, using the largest sample size of animals to date. Sneezing and rubbing counts recorded for 0–20 minutes showed stronger correlations than counts obtained over other time intervals with type 2 allergic parameters, such as serum OVA-specific IgG2a, the protein levels of splenic IL-4 and IL-5, and eosinophilic infiltration in the nasal tissue. We also analyzed the optimal time duration for symptom assessment and found that 0–20 minutes demonstrated a closer correlation with type 2 mediators than other time intervals. Significant correlations with allergic parameters were found for a duration of 5–20 minutes, but with a smaller correlation coefficient, suggesting that an interval of 0–20 minutes would be more appropriate for future experiments.
In a literature review, we found that some authors excluded the first few minutes after the last intranasal challenge from symptom scoring, perhaps to eliminate the effect of mechanical stimuli [6-9]. Our results demonstrated that the first 5-minute interval had the highest symptom score, which could have been affected by the mechanical stimuli of nasal instillation, whereas this interval showed the lowest correlation coefficients with serum total IgE, OVA-specific IgE, and nasal IL-4 mRNA levels.
Our work has some limitations. First, although we have analyzed various type 2 cytokines, additional type 2-associated mediators such as IL-13, periostin, or eosinophil cationic protein may be helpful to analyze. Second, if we had measured sneezing and rubbing counts for longer than 20 minutes after the last challenge, we might have presented stronger evidence for the association between inflammatory markers and sneezing and rubbing counts. Despite these limitations, our data provide strong evidence that sneezing and rubbing counts are well correlated with type 2 allergic parameters in the mouse model of AR. Furthermore, recording counts over a time interval of 0–20 minutes may be more effective for representing allergic symptoms in mouse models of AR.
ACKNOWLEDGMENTS
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (Ministry of Science and ICT, MSIT) (NRF-2016R1A2B4010407 to Ji-Hun Mo and NRF-2019M3E5D1A02069063 to Gwanghui Ryu), and Leading Foreign Research Institute Recruitment Program through NRF funded by the MSIT (NRF-2018K1A4A3A-02060572).
SUPPLEMENTARY MATERIALS
Supplementary materials can be available at https://doi.org/10.21053/ceo.2019.02005.
Supplementary Table 1.
Pearson’s correlation coefficients between sneezing and rubbing counts and allergic parameters
REFERENCES
1. Wang XD, Zheng M, Lou HF, Wang CS, Zhang Y, Bo MY, et al. An increased prevalence of self-reported allergic rhinitis in major Chinese cities from 2005 to 2011. Allergy. 2016; Aug. 71(8):1170–80.

2. Demoly P, Bousquet PJ, Mesbah K, Bousquet J, Devillier P. Visual analogue scale in patients treated for allergic rhinitis: an observational prospective study in primary care: asthma and rhinitis. Clin Exp Allergy. 2013; Aug. 43(8):881–8.
3. Almansouri HM, Yamamoto S, Kulkarni AD, Ariizumi M, Adjei AA, Yamauchi K. Effect of dietary nucleosides and nucleotides on murine allergic rhinitis. Am J Med Sci. 1996; Nov. 312(5):202–5.

4. Mo JH, Kang EK, Quan SH, Rhee CS, Lee CH, Kim DY. Anti-tumor necrosis factor-alpha treatment reduces allergic responses in an allergic rhinitis mouse model. Allergy. 2011; Feb. 66(2):279–86.

5. Samivel R, Kim DW, Son HR, Rhee YH, Kim EH, Kim JH, et al. The role of TRPV1 in the CD4+ T cell-mediated inflammatory response of allergic rhinitis. Oncotarget. 2016; Jan. 7(1):148–60.

6. Aoishi K, Takahashi H, Hato N, Gyo K, Yokota M, Ozaki S, et al. Treatment of allergic rhinitis with intranasal infusion of botulinum toxin type A in mice. Life Sci. 2016; Feb. 147:132–6.

7. Im YS, Lee B, Kim EY, Min JH, Song DU, Lim JM, et al. Antiallergic effect of gami-hyunggyeyeongyotang on ovalbumin-induced allergic rhinitis in mouse and human mast cells. J Chin Med Assoc. 2016; Apr. 79(4):185–94.

Fig. 1.
Sneezing and rubbing counts. (A) Sneezing and rubbing counts were significantly higher in the positive control group than in the negative control group. (B) Sneezing and rubbing counts at 5-minute intervals. The average count from the first 5-minute interval was higher than that of the consecutive 5-minute intervals. (C) Eosinophil count was significantly higher in the positive control group. ***P<0.001.
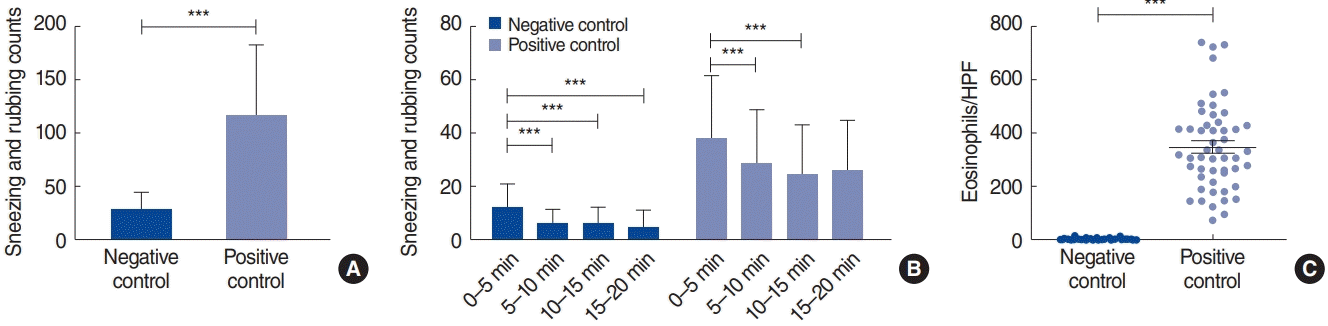
Fig. 2.
Correlation analysis between sneezing and rubbing counts and type 2 allergic parameters. (A) Type 2 immune response mediators were closely correlated with sneezing and rubbing counts for 0–20 minutes (P<0.001). (B) Summary of correlation coefficients according to the time interval. The correlation coefficient of nasal IL-4 mRNA levels was the highest among the type 2 markers. IgE, immunoglobulin E; OVA, ovalbumin; OD, optical density; IL, interleukin.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download