INTRODUCTION
Exposure to noise induces hearing loss, either temporary or permanent, according to the intensity of noise and the duration of exposure. Permanent threshold shift of hearing (permanent hearing loss) occurred by the derangement of the auditory organ, especially the outer hair cell (OHC) in the organ of Corti (
1). The mechanisms of death of OHC are understood as both of cellular necrosis and apoptosis (
2). Apoptosis is an active process of programmed cell death, but necrosis usually occurs as passive process (
2). In previous studies of morphological changes in OHC after noise exposure, swelling of OHC is mainly observed, and this finding is evidence of the necrosis. Persistent cellular damages of OHC, however, was also observed up to 30 days after brief exposure to noise (
2-
4). This persistent damage can be explained by an active process of programmed cell death with use of energy unlike cellular necrosis (
2-
4). Recently, the role of apoptosis in noise-induced OHC damage has been established in the early phase following noise exposure (
5,
6).
As for morphological changes in the nucleus and cytoplasm, apoptosis is considerably different from necrosis. DNA fragmentation, chromatin condensation and nuclear shrinkage can be observed in apoptosis by the nuclear staining with fluorescence microscopy (
7). A series of process of apoptosis is accompanied by degeneration of filamentous actin (F-actin) (
8). F-actin is a major cytoskeletal protein of OHC involving many physiological functions including motility, adhesion and maintenance of cellular shape (
9) and is distributed primarily in the cuticular plate and stereocilia of the hair cell. Previous studies have observed the changing pattern of F-actin and electrical potential of stereocilia following noise exposure (
8,
9). Changes in the nucleus precede the changes in F-actin in apoptotic cells, and signal molecule evoked by nuclear changes may effect on the changes in F-actin (
10).
In an animal model of noise-induced hearing loss (NIHL), a nuclear condensation was observed by the nuclear staining with propidium iodide (PI). These processes accompanied by the changes in F-actin suggesting the occurrence of the apoptotic process in the hair cells (
11,
12). Even in the apoptotic cells, the change in F-actin may be not severe in the early phase of damage and prominent in the late phase. Therefore, we could decide the stage of cell death according to the change in F-actin.
The aim of the present study is to observe the detailed OHC morphology according to the staining of PI and F-actin and to analyze them quantitatively along the whole length of cochlea. Because the morphological changes in OHC in the noise-exposed cochlear are remarkable at the basal turn of the cochlea corresponding to high frequencies, we have also analyzed the cellular changes and hearing loss according to the frequencies.
Go to :

MATERIALS AND METHODS
Materials
Six-week-old BALB/c mice (Orient Charles River Technology, Seoul, Korea) with normal Preyer's reflex and a normal hearing threshold, were used. All animal experiments were performed with food and water available ad libitum and with the approval of the Animal Care Committee of University of Ulsan College of Medicine. The care and use of the animals reported in the present study were in accordance with the guidelines of the Laboratory Animal Unit of the Asan Institute for Life Sciences.
Experimental instrument
White noise (300-10,000 Hz) was generated by a personal computer using Cool Edit 1.52 software and amplifier (R-399, INTER M, Seoul, Korea) and delivered through a speaker (290-8L, Altec Lansing, OK, USA) in a sound-proof booth. Planned noise in a booth was calibrated by a sound level meter (B&K, Narum, Denmark) at the center and the edge of a booth.
Anesthesia
When we collected the cochlea from mice and measured hearing threshold, mice were anesthetized by intramuscular injection of ketamine hydrochloride (30 mg/kg) and xylazine (2 mg/kg).
Measurement of basal hearing threshold
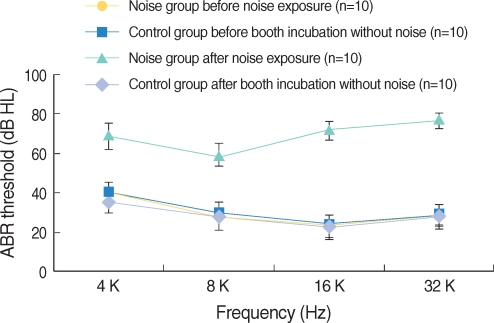
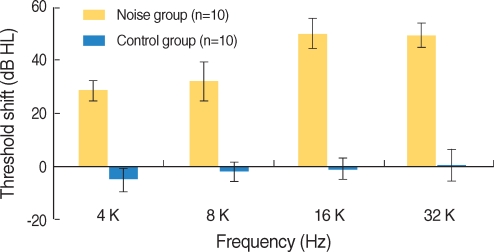
The hearing level of each mouse was determined by measuring ABR thresholds using auditory evoked potential workstation (Tucker-Davis Technologies, Alachua, FL, USA). Mice were anesthetized by intramuscular injection of ketamine hydrochloride (30 mg/kg) and xylazine (2 mg/kg), and both ears of each mouse were stimulated with ear probes sealed into the ear canal. The frequency-specific ABR in response to tone burst sound was recorded at 4, 8, 16, and 32 kHz. Sound stimuli were applied and the threshold was defined as the lowest intensity of stimulation that yielded a repeatable waveform based on an identifiable ABR wave V. Hearing threshold was measured the day after noise exposure.
Generation of NIHL
Mice were continuously exposed to 122 dB peak equivalent sound pressure level white noise (300-10,000 Hz, continuous tone) for 3 hr per day for 3 consecutive days in the experimental group. Mice in the control group were kept in the noise booth for 3 hr per day for 3 consecutive days without noise.
Immunofluorescent staining of hair cell
Immediately after the measurement of hearing threshold, mice were deeply anesthetized by intramuscular injection of ketamine hydrochloride (50 mg/kg) and xylazine (2 mg/kg), and both cochleae were removed from each mouse. The stapes were removed and a small hole was made in each cochlear apex with a fine pick. Fixative (4% formalin and 1% glutaraldehyde in 0.1 M sodium phosphate buffer) was infused and the cochleae were immersed in fixative for 48 hr at 4℃. After washing with phosphate-buffered saline (PBS), the outer bony wall of each cochlea and the tectorial membrane were gently removed, and the organ of Corti was harvested with fine forceps beginning from the apex.
Fluorescein isothiocyanate (FITC)-phalloidin (1 µg/mL, Sigma, St. Louis, MO, USA) was used for F-actin staining. We mounted the specimen on slides after reaction with FITC-phalloidin for 1 hr at 4℃ in a darkroom and rinsing it with PBS.
For the nuclear staining, PI (4 µg/mL, Molecular Probes Inc.) was used. PI showed fluorescence when inserted in DNA in nucleus.
Fluorescence microscopic examination
We observed a double-stained cell layer by confocal microscopy, which was able to examine fluorescently. The PI-labeled nuclei (red fluorescence) and FITC labeled F-actin (green fluorescence) were in one field of view simultaneously scanned in the cochlear section of interest and counted number of stained OHC.
When we observed cellular tissue, we made coverage come into sight in one field of view of a region and examined after dividing into 16 consecutive regions from the apex to the base of the cochlea. While the cells were fixed, every cell was stained by PI. Therefore, typical findings of PI staining corresponding to apoptosis were increased fluorescence intensity and prominent nucleus compared with surrounding cells. As apoptosis proceeded, chromatin and nucleus condensed. PI (+) was nuclear condensation featured by nuclear condensation with an increase in PI fluorescence staining. PI (-) was nuclear swelling characterized by enlargement of the nucleus, and a decrease in PI fluorescence intensity. PI (+) is evidence for the presence of apoptosis, and PI (-) denotes absence of apoptosis (
11,
12). FITC (+) was intact F-actin; stereocilia was stained well with FITC and had no morphologic change. FITC (-) was breakdown of F-actin; stereocilia was not stained with FITC or had abnormal morphology. We conducted the quantitative analysis of F-actin-staining and PI-staining cells at each region. At this time, we counted the staining condition of OHC according to FITC (+) PI (-), FITC (+) PI (+), FITC (-) PI (+), FITC (-) PI (-) (
Fig. 1). We defined a FITC (-) PI (-) cell in the course of staining as the missing cell.
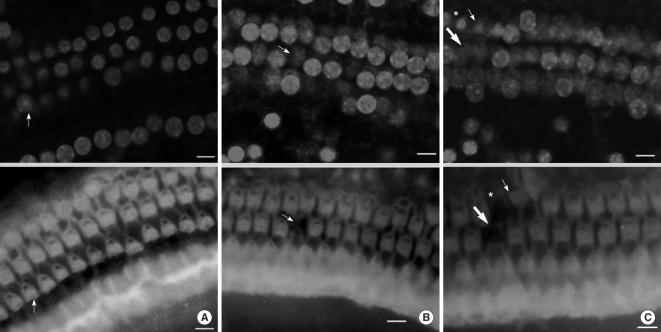 | Fig. 1Images of immunoreactivity for PI and FITC-phalloidin in the organ of Corti of a noise-exposed mouse (×400). (A) Apical turn. There shows a nuclear condensation in a fluorescent image for PI (PI+, arrow, upper panel). In an image for FITC-phalloidin, the corresponding cell shows normal appearance (FITC+, arrow, lower panel). Other cells are normal live cells (FITC+, PI-). (B) Middle turn. There is a cell with no nuclear staining (arrow, upper panel) and no fluorescence for FITC-phalloidin (arrow, lower panel). This cell is considered as missing. (C) Basal turn. Nuclear condensation (PI+, arrow, upper panel) with an intact structure (FITC+, arrow, lower panel) was noted in one cell. Adjacent cells (asterisk in both panels) show nuclear condensation and a deformed structure (FITC-, PI+). The other cells (thick arrow in both panels) have no nucleus and show deformed structures (missing). Scale bar: 10 µm. 
|
Statistical analysis
Statistical analysis of the data was performed using SPSS 12.0 K (SPSS Inc., Chicago, IL, USA). We used the paired t test for analysis of changes in hearing threshold following noise exposure and the Student t test for comparison of hearing threshold between the experimental and control groups.
Go to :

DISCUSSION
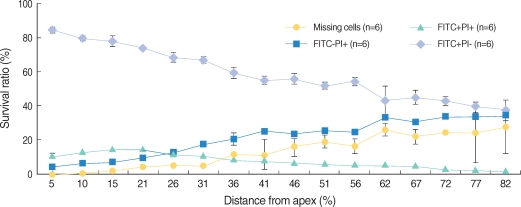
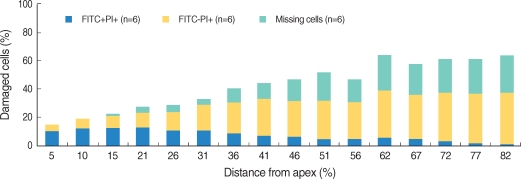
In this study, we observed morphologic changes in OHC of mice after noise exposure including apoptosis. The findings, such as chromatin condensation and hyper-staining, represent the process of apoptosis of OHC, and in this study we observed degradation of F-actin simultaneously during apoptosis so that we could find more information about the mechanism of cellular apoptosis. We can summarize the observation of morphological changes of OHC as follows: 1) Noise exposure induced loss of OHC, and the number of lost OHC increased from the apex to the base, 2) There existed cells which were in the process of apoptosis among OHC which remained without loss and the number of such cells also increased toward the base, 3) Cells in the cochlea apex continued to have F-actin, while the rate of degradation of F-actin increased toward the base, 4) The characteristics of cellular damage according to 2) and 3) were evident around the region corresponding to 60% of the whole cochlea, and the degree of cellular damage around this area was similar to that distal to this region.
OHC is the weakest cell against noise exposure in the cochlea, and the morphological changes in OHC are known to be pathophysiological key features of the results of noise trauma to the cochlea, and these changes are more remarkable in the base than the apex of the cochlea (
1,
3). In this study, the more missing cell increased, the closer it approached to the base. The aspect of apoptosis was more evident at the base of the cochlea and this phenomenon is proper considering the process of apoptosis accompanied the loss of OHC during death process of the cell. In conclusion, the degree of damage to OHC increased toward the base of the cochlea.
Previous report has shown that the degree of F-actin staining evidently change according to the degree of changes in the nucleus with PT staining. In other words, the degree of F-actin stains ambiguously in the cells which show an increase in degradation and severe condensation of chromatin. Lysis of cytoskeletal proteins and collapse of cell structure are key features of apoptosis of cells (
8,
9). F-actin is resolved by proteases. Especially caspases are important (
14). The changes in F-actin are mediated by the signal of apoptosis and the affected process of apoptosis (
10). In the process of apoptosis, they are well known as changes in F-actin which happen in the early stage of the cell cycle (
15,
16).
In addition, we intended to investigate the relation between morphologic changes in OHC following noise exposure and the total length of the cochlea. PI (+) cells implied that apoptosis increased gradually from the apex to the base of the cochlea. The former situation was led by FITC (-) PI (+). The number of FITC (+) PI (+) cells was larger than that of FITC (-) PI (+) in the apex, but the number of FITC (-) PI (+) cells increased more approaching the base. From the above-mentioned results, it is supposed that the closer approach to base of cochlea, the more expansively and rapidly apoptosis proceed. The number of PI (+) cells was in the region corresponding to 60% of the total length of the cochlea and from this point to the base, the count of PI (+) cells was similar. In other studies which quantitatively analyzed the damage to OHC, the damaged OHC was distributed in 60-70% of the total length of the cochlea markedly.
The cellular apoptosis of the damaged OHC spread to both of the apical and basal turns of the cochlea with time. However, apoptotic cells were more prominently distributed in the base of the cochlea (
6,
17). There was specifically damaged site by noise in the total length of the cochlea and the active apoptotic site mainly moved to the base of the cochlea. Damage to OHC repeatedly induced at specific sites of the cochlea by three noise stimuli with time intervals. When noise stimulation was repeated, OHC at specific sites were less damaged because they were already destroyed. On the other hand, apoptosis and damage by noise more increased in OHC located in the vicinity of the base of cochlea than the specific site. Consequently, after three noise stimuli, OHC was damaged similarly and constantly. We need to compare apoptotic patterns through the observation of OHC immediately, and 1 and 2 days after noise exposure in order to prove this hypothesis.
The frequency-specific ABR in response to tone burst sound was recorded at 4, 8, 16 and 32 kHz in order to compare hearing thresholds before and after noise exposure in this study. Increase in hearing threshold was more prominent at 16 and 32 kHz than at 4 and 8 kHz. Hearing threshold increased from 4 to 16 kHz gradually, and changes in hearing threshold were similar between 16 and 32 kHz.
The Digital Cytocochleogram in C57BL/J mice most similar with the relation of hearing loss patterns by noise and the total length of organ of Corti in BALB/c mice with the rest of the resources of the MCD is posted on the MCD website, currently located at
http://mousecochlea.ccgb.umn.edu (
17). In this database, 16 and 32 kHz correspond to 58.8% and 75.8% distance from the apex of the cochlea, respectively. Cell damage in the real cochlea between 16 and 32 kHz maintained a similar plateau of rise in hearing threshold. Therefore, there is a similar trend between the extent of practically measured hearing loss and microscopically examined damage to OHC.
There is no treatment of NIHL, excluding avoidance. There have been many researches on blocking active signal delivery of apoptosis and on suppressin missing of OHC. Because structural features of FITC (-) PI (+) in apoptosis of OHC differ from those of FITC (+) PI (+), it is thought that responses to treatment differ from each other.
Close investigation of morphological features at each step of cell death could help to determine the exact time of treatment for apoptotic process and to estimate the response to treatment.
In conclusion, we could show the morphological features of OHC through the process of apoptosis after noise exposure with the double staining method. The degree of loss and apoptosis of OHC, especially with degraded F-actin, increased toward the base of the cochlea until 60% of the whole length and evenly distributed subsequently. We found that the increase of hearing threshold corresponded with the degree of damage to OHC. We believe that morphologic features of apoptosis observed in this study would help to treat NIHL.
Go to :






 Citation
Citation Print
Print


 XML Download
XML Download