INTRODUCTION
Orbital exenteration is the removal of the eyeball and orbital contents [
1], and may include the walls of the eyelids and orbital bones. This procedure is disfiguring and destructive. Generally, orbital exenteration is used to treat tumors and, rarely, inflammation and trauma [
2,
3]. The main reasons for orbital exenteration are tumors originating from the orbital structures, including basal cell, squamous cell, and sebaceous cell carcinomas [
4]. In head and neck surgery, orbital exenteration is commonly used to treat skin, nasal cavity, and paranasal sinus malignancies. Orbital exenteration can be further subdivided into total, subtotal, and enlarged types [
5].
Postoperatively, orbital exenteration causes aesthetic and psychological problems. Therefore, this procedure is difficult for both patients and doctors. The most important decision is whether to perform primary or secondary reconstruction, as healing of the orbital defect is dependent on the reconstructive method. The goals of reconstruction are to detect recurrent disease, restore the boundaries between the orbit and surrounding cavities, and to produce an acceptable aesthetic outcome.
Several methods are available for reconstructing the defect resulting from exenteration, ranging from cavity healing with granulation tissue to the use of microsurgical flaps to reconstruct the cavity [
6,
7,
8]. In this paper, we describe the use of the temporalis muscle flap to reconstruct the orbital exenteration cavity and discuss the clinical indications for orbital exenteration.
Go to :

RESULTS
The eye was not the origin of the primary tumor in all cases. Histological examinations revealed that the sample included six patients with skin cancers and seven with nasal cavity and paranasal sinus cancers. The most common cancer was squamous cell carcinoma. Of these, four originated in the skin and three in the nasal cavity and paranasal sinuses (
Tables 1,
2).
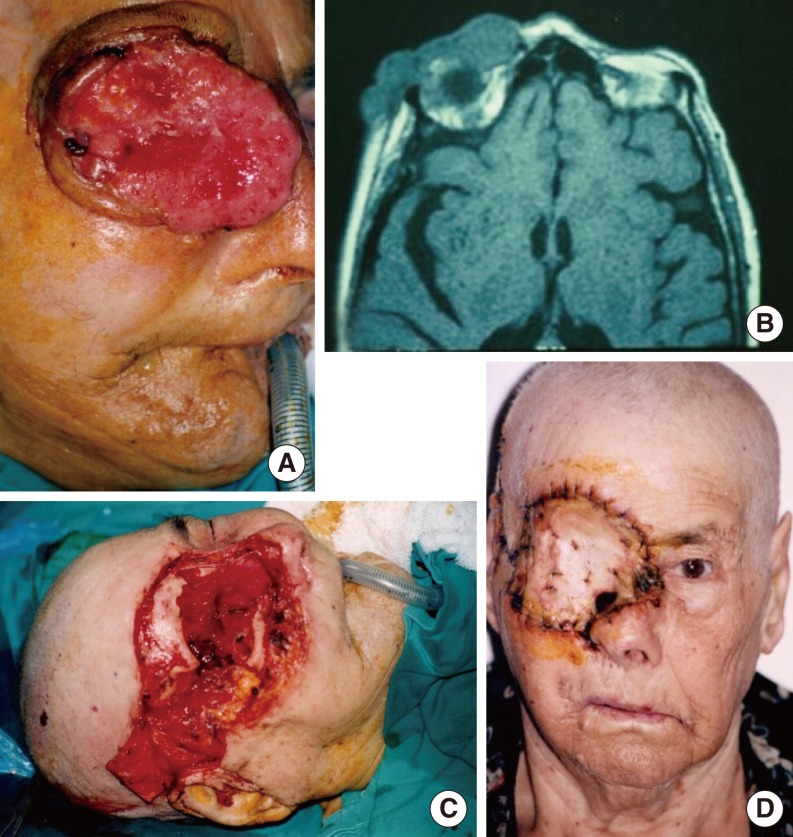
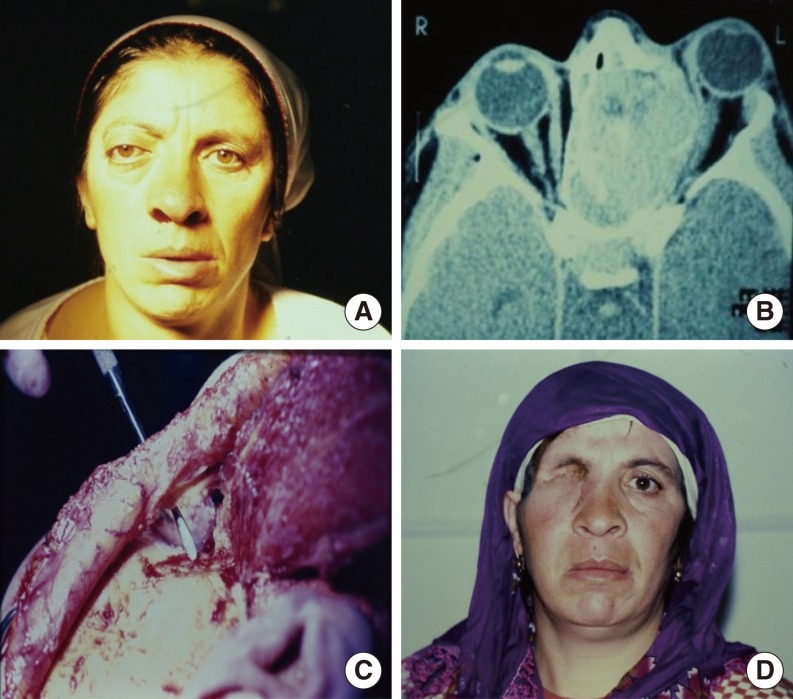
In the orbit, the tumors most frequently involved the periorbital soft tissues (n=13), followed by the anterior compartment of the orbit (n=10).
Paralysis of the frontal branch of the facial nerve was seen in three patients. An orbital fistula developed in two cases. No visible defects were present in the muscle flap donor sites (
Table 3).
In our series, four patients had local recurrence. These patients underwent subsequent surgery to close the defect using a midline forehead flap and a cheek flap. These patients were followed for at least 2-5 years. A maxillectomy, ethmoidectomy, or craniofacial resection was performed as necessary. If there was invasion of the orbital wall, the bony structures were also resected en bloc.
Three of the patients died with recurrence involving the skull base and the cavernous sinus of the brain. Recurrences were diagnosed radiologically in these patients. Two patients died of systemic metastases and five died for other reasons (infarction, diabetes complications, and a vehicular accident). Three patients are alive and still being followed.
Go to :

DISCUSSION
Previously, orbital exenteration was indicated for invasion of the periorbital region; however, it is currently used for tumors invading the orbital apex or sphere.
The decision to perform orbital exenteration when orbital involvement is suspected is difficult. Invasion of the fat and muscles of the orbital apex and infiltration of the conjunctiva or sclera are absolute indications for orbital exenteration.
CT and magnetic resonance imaging (MRI) are not sufficient for a definitive diagnosis of invasion in patients without eye movement limitation and proptosis. Therefore, frozen section examination should be performed during the operation. The tumor histology is important, as exenteration of the orbit is less often necessary in cases of esthesioneuroblastoma, adenocystic low-grade carcinoma and benign tumors, adenocarcinoma, and squamous cell carcinoma [
9].
The purpose of the reconstruction is to restore the orbital tissues and to provide an acceptable aesthetic appearance, while allowing fot the detection of recurrent disease.
When considering orbital exenteration, an ophthalmologist, prosthodontist, radiologist, and radiation oncologist should be consulted preoperatively. Patients and relatives should be informed. After these consultations, the surgeon will decide whether to perform primary or secondary reconstruction. Reconstruction methods include temporalis muscle transposition; the use of midline forehead flaps, dermal grafts, dermis fat grafts, and split skin grafts; including globe-sparing exenteration, eyelid-sparing techniques, and spontaneous granulation [
9,
10].
Primary reconstruction is performed during the initial exenteration operation. It promotes healing, provides a better aesthetic appearance, and enables activities such as bathing, but it can hide recurrences. Postoperative radiotherapy can impair the vascularization of the flap and cause orbital dehiscence [
11]. When dural resection and reconstruction are performed in addition to orbital exenteration, primary reconstruction is mandatory in order to avoid the risk of meningitis.
Some surgeons prefer to allow spontaneous healing of the orbital cavity. This approach leads to secretions, mucus retention, and maceration. Sound resonance is worse. However, recurrence can be diagnosed early when the cavity is exposed for secondary reconstruction. The average healing time is 14 weeks, but healing can take up to 6 months [
12]. Some case series have found a high rate of sino-orbital fistulas following spontaneous granulation of exenterated orbital cavities (17/25 cases) [
7]. Covering the orbit with split-thickness skin grafts and local flaps speeds the healing process. Many regional flaps (temporalis muscle, midline forehead, frontal, or temporo-parietal fascial flaps) or free flaps (rectus abdominis, latissimus dorsi, radial forearm, and lateral arm flaps) are used for this purpose.
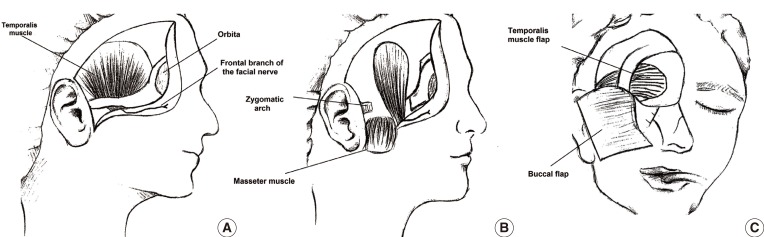
The orbit is closed with temporal flaps, the eyelids, or a partial- or full-thickness skin graft. Muscle only, rather than fascia, should be used to improve the nutrition of the skin graft. The volume of the temporalis muscle used is smaller than with other muscles. It covers the orbit from the lateral orbital wall to the nose like a curtain. Recurrence in the orbit, skull base, and nasal cavity can then be evaluated via nasal endoscopy. This procedure also avoids the shortcoming of primary reconstruction hiding recurrence [
13].
CT and MRI are useful modalities for evaluating the characteristics, anatomical site, and extent of suspected orbital involvement. CT and MRI can also be used during in follow-up to monitor recurrences. A local flap that covers the orbit will not hide recurrences [
10].
The most common complication is a defect in the temporal region. With most local and free flaps, donor defects can occur, affecting the patient's aesthetic appearance. In our cases, the flaps were prepared from the posterior 1/3 or 1/4 of the temporalis muscle, which prevents a donor defect.
Another important consideration is the risk of injury to the frontal branch of the facial nerve on the temporalis muscle during elevation of the skin. This nerve is located between the two layers of temporoparietal fascia in the fat tissue. When the skin flap includes the fat tissue over the internal layer of the fascia, it is not at risk. While preparing the flap, injury may occur as a result of stretching the nerve, but this is a reversible condition. In this study, three patients had frontal facial paralysis.
Free flaps extend the nature of the operation and an additional team is needed. By contrast, the temporal flap is adjacent to the orbit, and requires only an incision in the scalp, which will be hidden by the scalp, and is easy to elevate.
Chemotherapy and radiotherapy should be combined in order to treat advanced disease for palliation. Osteoradionecrosis, osteomyelitis, and eye complications can be seen in follow-up.
Follow-up for recurrences in this study was performed in two ways: endoscopic examination of the nasal cavity and radiology. The temporalis muscle flap is not as thick as free flaps, and the recurrence can be diagnosed readily by endoscopic nasal examination. However, this is not always enough. Radiologic examination of the skull base and orbital cavity can be used for recurrences and follow-up as well. MRI and CT help to diagnose brain and skull base involvement respectively. Recently fluorodeoxyglucose positron emission tomography/CT scan has been used for recurrences and distant metastasis. In this study, three patients died with recurrences while two patients died of systemic metastases.
After orbital reconstruction, various ocular prostheses can be used as requested by the patient, including hydroxyapatite implants, polymethylmethacrylate prostheses, and bone-implanted dentures [
14,
15].
The temporalis muscle flap was preferred for reconstruction in this study. This flap has a good vascularization and it is close to the defect region. It is easy to perform and adequate in terms of length, width and volume. Additionally, it has better vascularization than dermal and skin grafts and results in a better cosmetic appearance. It has a short healing time compared to secondary wound healing. Also it has a short operation time and less visible scar in the donor site compared to the free flap technique. This is a thin flap, making it easy to follow-up for recurrences with nasal endoscopy. The temporalis muscle flap should be preferred for primary reconstruction of orbital exenteration defect.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download