Abstract
Objectives
Postintubation tracheal ruptures (PTR) are rare but cause severe complications. Our objective was to investigate the tracheal pattern of injury resulting from cuff inflation of the tracheal tube, to study the two main factors responsible for PTR (cuff overinsufflation and inapplicable tube sizes), and to explain the context, why small women are particularly susceptible to PTR.
Methods
Experimental study performed on 28 fresh human laryngotracheal specimens (16 males, 12 females) within 24 hours post autopsy. Artificial ventilation was simulated by using an underwater construction and a standard tracheal tube. Tube sizes were selected according to our previously published nomogram. Tracheal lesions were detected visually and tracheal diameters measured. The influence of body size, sex difference and appropriate tube size were investigated according to patient height.
Results
In all 28 cases, the typical tracheal lesion pattern was a longitudinal median rupture of the posterior trachea. Appropriate tube sizes according to body size caused PTR with significantly higher cuff pressure when compared with oversized tubes. An increased risk of PTR was found in shorter patients, when oversized tubes were used. Sex difference did not have any significant influence.
Conclusion
This experimental model provides information about tracheal patterns in PTR for the first time. The model confirms by experiment the observations of case series in PTR patients, and therefore emphasizes the importance of correct tube size selection according to patient height. This minimizes the risk of PTR, especially in shorter patients, who have an increased risk of PTR when oversized tubes are used.
Iatrogenic postintubation tracheal ruptures (PTR) are rare but severe complications are estimated to occur in 0.05%-0.37% of intubations [1]. Its importance is due to the high overall mortality rate of 22% [1]. In literature, there are few case studies with isolated patients, the largest involving 30 patients [23]. PTR typically occur in emergency situations during multiple forced intubation attempts in combination with inexperience of the health professionals [14]. However, PTR can also occur during elective surgeries [5]. The percentage of women affected is 86.2%, who are on average over 50 years old [14]. The smaller body size of females [6] and children [7] is assumed to be a predisposing factor when relatively large tubes are selected [89]. These oversized tubes are placed too far distally and cause PTR in the lower third of the trachea [10]. Almost all PTR described in the literature were found on the posterior wall of the trachea, usually longitudinally along the right tracheal side and rarely centred [1112].
Since evidence based data for the maximum allowable cuff pressure has not been available to date, the recommended maximum cuff pressure is 30 mbar. Cuff pressures exceeding 30 mbar reduce blood flow, and cause complete blockage over 45 mbar [13]. Both may damage mucosal tissue leading to tracheal stenoses, trachea-oesophageal fistula or tracheal perforation [14]. Nevertheless, 10% of all ventilated patients experience ischemic damage of the tracheal mucous membranes despite high volume, low pressure cuff tubes being used [14]. Manual regulation of the tube cuff leads to the recommended pressure range in <30% of cases, even for experienced emergency physicians [5151617]. Very few reports in literature have investigated the effect of the tracheal tube cuff on the tracheal membrane. In vivo animal studies have shown microscopic alterations of the tracheal wall depending on the cuff shape [18]. In vitro studies used a tracheal cadaver [19] or model trachea [20] to determine mucosal pressures. These studies used the recommended volume or pressure according to the manufacturers' instructions. The exact mechanism of PTR and the values of pressure rupture resistances continue to remain uncertain. The most common hypothesis for PTR is overinsufflation of the cuff, usually in connection with the use of oversized tracheal tubes [5891116212223], both of which can be prevented. Our research group has previously produced evidence for appropriate tube size selection according to a body size based nomogram [24]. In literature, there is neither an experimental model for PTR, nor data on tracheal pressure rupture resistance. The aim of the present study was to develop an experimental in vitro model of human tracheal specimens in order to study tracheal injury patterns resulting from tracheal intubation tubes, and to study the two main factors responsible for PTR, cuff overinsufflation and inapplicable tube sizes. We also investigated the influence that body size and sex difference has on the risk of tracheal ruptures, as the smaller body size of females [6] and children [7] is assumed to be a predisposing factor.
An experimental study was performed on 28 fresh human laryngotracheal cadaveric specimens, including the main bronchi (16 males, 12 females). The study was approved by the Institutional Ethics Authorities and there was written informed consent for all post mortem examinations. The study included all specimens of autopsies performed between September 2011 and September 2012 in a tertiary hospital. Excluding criteria were existing or previous tracheostoma, malignancies or other suspicious diseases involving the trachea and any other criteria which required further investigation. The patients' characteristics (age, height, gender, tracheal diameter) were recorded in Table 1. The laryngotracheal specimens were preserved in a fridge (4°C). The experiments were performed within 24 hours post autopsy. During preparation of the laryngotracheal specimens, the structure of the esophagus at the trachea's posterior wall was relaxed in many cases, with the oesophagus partially or completely peeled off. The outer saggital and coronal tracheal diameters were measured 2 cm below the cricoid cartilage using a calliper ruler.
Artificial ventilation was simulated using an underwater construction by connecting the trachea and main bronchus with an artificial lung, which consisted of small tracheal tubes closed by a commercial air balloon (Fig. 1). The water temperature was 25°C. A tracheal tube was placed subglottically and fixated to the laryngotracheal cadaveric specimen using sutures. The tracheal tube was connected with a respirator to ventilate the artificial system (ventilation modus; ventilation frequency 18/minute ventilation volume 71/minute). The tube cuff pressure was adjusted using a gaged digital manometer (P40.2, SIKA, Kaufungen, Germany). The cuff pressure was increased by 100 mbar every 5 minutes until a tracheal lesion or bubble escape was detected. Before the cuff pressure was increased, the pressure was completely released for 1 minute. The saggital and coronal outer tracheal diameters were determined after each increase of pressure. The localization of the tracheal lesion was detected visually. The pressure rupture resistance was defined as the measured pressure at the time of tracheal lesion detection.
We used standard high volume, low pressure, cuffed tracheal tubes (Safety Flex, Mallinckrodt, Covidien, Neustadt/Donau, Germany) with a tube size of 6.0-10.0 internal diameter. The tube size was applied according to the patients' height and determined by the nomogram [24]. For subclassification, the 28 laryngotracheal cadaveric specimens were divided by patient height into two equally sized, height dependent groups, one 'taller' and one 'shorter' (14 cadavers in each group). In 7 cadavers of each of the two groups, we used the applicable tube size according to the nomogram, and in the other 7 we used an oversized tube, which was one size bigger than recommended by the nomogram (e.g., 9.0 instead of 8.0).
Statistical analyses were performed using SPSS ver. 17 (SPSS Inc., Chicago, IL, USA). Differences in nonnormally distributed data were examined using the Mann-Whitney U-test. The probability of a tracheal rupture depending on pressure rupture resistance was calculated using the Kaplan-Meier analysis, represented as 1 minus the probability of survival. The confidence interval (CI) was 95%. Different groups were compared using the log-rank test. Statistical significance was defined as P≤0.05 in all tests.
In all tracheal specimens, a longitudinal tear occurred in the middle of the pars membranacea. The extension of the perforation corresponded to the cuff size. The rupture occurred in the pressure increase during cuff inflation phase.
The pressure rupture resistance of the 28 specimens investigated at the time of the tracheal rupture ranged between 700 and 1,300 mbar (Fig. 2A). The relative increase in the sagittal and coronal tracheal diameter exhibited approximately equal values. Therefore, we present as an example the diagram for the coronal diameter (Fig. 2B). The diagram shows a logarithmic trend with increasing pressure rupture resistance. For further investigation of PTR risk, we studied the influence of body height, sex difference, and appropriate tube size according to the pressure rupture resistance.
The pressure rupture resistance during tracheal ruptures was a median of 1,200 mbar in suitable and 800 mbar in unsuitable tube sizes, according to the patients' body height. Unsuitable tubes had a significantly lower pressure rupture resistance (P< 0.001) (Fig. 3A). The relative increase in the tracheal diameter both coronary and sagitally was increased using unsuitable tubes compared with suitable oversized tubes (data not shown). The probability of PTR rises with increasing pressure rupture resistance (Fig. 3B) and differs significantly with both suitable and unsuitable tubes (P<0.001).
For example, the probability of a tracheal rupture using a cuff pressure of 700 mbar and a suitable tube is 7% (95% CI, 0 to 21), whereas for an unsuitable tube the probability is 23% (95% CI, 0 to 46). When using a cuff pressure of 1,000 mbar, the probability increases to 29% (95% CI, 5 to 52) for suitable tubes and to 85% for unsuitable oversized tubes (95% CI, 65 to 100).
The tracheal specimens were divided into two equally sized groups (n=14) depending on the patients' height. The first group included the specimens of patients ≤163 cm (shorter height) and the second group the specimens of patients ≥164 cm (taller height). A trend toward an increased risk of tracheal ruptures was found in shorter patients (P=0.069), which was negligible when applicable tube sizes were used (Fig. 4A). Shorter patients were significantly more susceptible to tracheal ruptures when unsuitable tubes were used (P=0.005) (Fig. 4B).
The tracheal specimens were removed from 16 males and 12 females. The tracheal pressure rupture resistance did not show any statistical difference between the two groups (P=0.565).
The exact mechanism of PTR continues to remain uncertain. In literature, there are few case studies, the largest involving 30 patients [23]. The most common hypothesis for PTR is overinsufflation of the cuff, usually in connection with the use of oversized tracheal tubes as PTR are most often found in women [5891116212223]. There is, however, no data on tracheal pressure rupture resistance in literature. We therefore developed an experimental in vitro model in the current study, designed to measure pressure rupture resistances. We also investigated the extent to which pressure rupture resistance depended on tube size. We used fresh human laryngotracheal specimens within 24 hours post autopsy. Since there has been no official, evidence based guidelines for tube size selection, a suitable or unsuitable tracheal tube has been determined by a nomogram published previously by our research group [24]. The nomogram is based on patient height and tracheal diameter. In half of the specimens, a proper fitting tube was used, whereas in the other half an oversized (one size bigger) tube was used (e.g., 9.0 instead of 8.0).
In our study, the pressure rupture resistance measurements ranged between 700-1,300 mbar. These values are far above the generally recommended upper limit of 30 mbar for cuff blocking, which can already cause mucosal tissue damage, crust formation and lead to tracheal stenosis [14]. However, several studies indicate that manual regulation of the tube cuff without a pressure gauge leads to the recommended pressure range in <30% of cases [5151617]. In most cases, even experienced emergency physicians produced cuff pressures above 100 mbar [16]. The exact pressure values could not be specified because the cuff pressure gauge devices can only measure up to 100 mbar. Since in most cases, the actual cuff pressure in manual regulation was above 100 mbar, some cases may have reached cuff pressures of 1,000 mbar. The actual pressure is therefore certainly underestimated. Our diagram investigating coronal diameter and pressure rupture resistance showed a logarithmic trend of the cuff pressure with a low gain of the tracheal diameter above 100 mbar, despite greatly increasing cuff pressure (Fig. 2B). According to Boyle's law, a small volume gain leads to a strong increase in cuff pressure (pressure and volume are constant) whereas in practice, pressure values of 1 bar may be reached. Changes of the posterior tracheal wall, such as scars, alter the properties of the pars membranacea (e.g., elasticity) and may therefore also contribute to PTR at lower pressure rupture resistances. Additionally, nitrogen oxide and other volatile anesthetics diffuse into air-filled cavities and further increase the cuff pressure due to permeability of the cuff tube [25]. A limitation of the cuff pressure (e.g., maximum cuff pressure of 50-100 mbar) could prevent overinsufflation, i.e., due to overpressurised cuff valves. These values are arbitrary. A limit of lower values to around 30 mbar impairs the filling of the cuff. Another possibility would be automated cuff pressure modulation [26].
The risk of tracheal ruptures rises with increasing pressure resistance and is represented using one minus survival by the Kaplan-Meier analysis (Fig. 3). The size range or suitability of the tube plays a crucial role. Unsuitable large tubes had significantly lower resistance rupture pressure compared with applicable tubes. This is caused because unsuitable oversized tubes reach higher cuff volumes compared with applicable tubes at equal pressure ratios. In clinical observations, an increased risk of tracheal ruptures was found in shorter patients [16]. In our study we also observed this tendency when we divided the patients into two equally sized groups depending on their heights. The cutoff for this subclassification in our study was 164 cm, which is consistent with case series of PTR patients [1127] including all patients less than 165 cm.
However, when we considered only the specimen with suitable tube sizes, we found an equal median pressure in both groups (Fig. 4A). Shorter patients were nevertheless significantly more susceptible to tracheal ruptures when unsuitable tubes were used (Fig. 4B), which may explain the clinical reports. The subclassification by gender was not identical to the subclassification according to patient height. In the group of shorter patients, three men were included, whereas the group of taller patients included no females. The pressure rupture resistance did not demonstrate any statistical difference with respect to females. The increased occurrence of PTR in females and shorter patients may be caused by unsuitably oversized tubes according to patient height [28].
Another important aspect of postintubational tracheal ruptures is the different cuff design and tube coat, which have scarcely been studied. The cuff length differs between manufacturers. A longer cuff is restricted by a larger contact surface, which may alter the elasticity of the posterior tracheal wall and be predisposed to tracheal ruptures. In this study, we used high-volume, low-pressure, cuffed tracheal tubes made of silicone with a round or cylindrical shape. These tubes have a reduced risk of pressure induced mucosal damage compared to the previously used high volume, low pressure, cuffed tubes made of rubber. The recently available conical shape is designed to ensure a better cuff seal on the tracheal wall. Recent studies have investigated self-expanding, foam shaped endolaryngeal tubes, which may cause significantly less tracheal injuries [29].
The tracheal rupture in the middle of the posterior tracheal wall in this experimental model can be explained by the partial or total separation of trachea and esophagus resulting from the preparation. This preparation was required for the local and early detection of posterior tracheal tears and for determining the increasing tracheal diameter. The increased risk of tracheal ruptures after esophagotomy or mobilization of the esophagus support this hypothesis [30]. Anatomically, the esophagus is located posterior to the trachea with a slight shift to the left. This explains the discrepancy between the centered longitudinal posterior tracheal wall ruptures in vitro and the right-sided in vivo findings [111223].
In the present study, only a limited number of in vitro preparations were investigated in an artificial model. This systematic study provides evidence for tracheal resistance rupture pressure. Larger specimen numbers may allow more comprehensive conclusions. In contrast to a high absolute pressure, an experimental setup at a lower pressure for several hours is conceivable. The laryngotracheal specimens were prepared postmortem, and were therefore difficult to apply to physiological conditions. Alterations of the tissue occur both as a result of death and specimen preparation, and have a significant influence on the stability of structures. Animal models may therefore have advantages [18]. The influence of cuff geometry and foam, sponge-filled cuffs would then also be able to be investigated.
In conclusion, this experimental model provides information about tracheal patterns in PTR for the first time. The model confirms by experiment the observations of case series in PTR patients, and therefore emphasizes the importance of correct tube size selection according to patient height. This minimizes the risk of PTR, especially in shorter patients, who have an increased risk of PTR when oversized tubes are used.
ACKNOWLEDGMENTS
We are very thankful to Dr. Ukrow (Department of Pathology at Unfallkrankenhaus Berlin, Charité Medical School, Berlin, Germany) for providing and preparing the laryngotracheal specimens.
References
1. Minambres E, Buron J, Ballesteros MA, Llorca J, Munoz P, Gonzalez-Castro A. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg. 2009; 6. 35(6):1056–1062. PMID: 19369087.
2. Conti M, Pougeoise M, Wurtz A, Porte H, Fourrier F, Ramon P, et al. Management of postintubation tracheobronchial ruptures. Chest. 2006; 8. 130(2):412–418. PMID: 16899839.

3. Cardillo G, Carbone L, Carleo F, Batzella S, Jacono RD, Lucantoni G, et al. Tracheal lacerations after endotracheal intubation: a proposed morphological classification to guide non-surgical treatment. Eur J Cardiothorac Surg. 2010; 3. 37(3):581–587. PMID: 19748275.

4. Chen EH, Logman ZM, Glass PS, Bilfinger TV. A case of tracheal injury after emergent endotracheal intubation: a review of the literature and causalities. Anesth Analg. 2001; 11. 93(5):1270–1271. PMID: 11682412.

5. Lim H, Kim JH, Kim D, Lee J, Son JS, Kim DC, et al. Tracheal rupture after endotracheal intubation: a report of three cases. Korean J Anesthesiol. 2012; 3. 62(3):277–280. PMID: 22474557.
6. Wagner A, Roeggla M, Hirschl MM, Roeggla G, Schreiber W, Sterz F. Tracheal rupture after emergency intubation during cardiopulmonary resuscitation. Resuscitation. 1995; 12. 30(3):263–266. PMID: 8867716.

7. Schedlbauer EM, Todt I, Ernst A, Seidl RO. Iatrogenic tracheal rupture in children: a retrospective study. Laryngoscope. 2009; 3. 119(3):571–575. PMID: 19160397.

8. Weiss M, Dullenkopf A, Gysin C, Dillier CM, Gerber AC. Shortcomings of cuffed paediatric tracheal tubes. Br J Anaesth. 2004; 1. 92(1):78–88. PMID: 14665558.
9. Randestad A, Lindholm CE, Fabian P. Dimensions of the cricoid cartilage and the trachea. Laryngoscope. 2000; 11. 110(11):1957–1961. PMID: 11081618.

10. Medina CR, Camargo Jde J, Felicetti JC, Machuca TN, Gomes Bde M, Melo IA. Post-intubation tracheal injury: report of three cases and literature review. J Bras Pneumol. 2009; 8. 35(8):809–813. PMID: 19750335.
11. Marty-Ane CH, Picard E, Jonquet O, Mary H. Membranous tracheal rupture after endotracheal intubation. Ann Thorac Surg. 1995; 11. 60(5):1367–1371. PMID: 8526628.
12. Sippel M, Putensen C, Hirner A, Wolff M. Tracheal rupture after endotracheal intubation: experience with management in 13 cases. Thorac Cardiovasc Surg. 2006; 2. 54(1):51–56. PMID: 16485190.

13. Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. Br Med J (Clin Res Ed). 984; 3. 288(6422):965–968.

14. Guyton D, Banner MJ, Kirby RR. High-volume, low-pressure cuffs. Are they always low pressure. Chest. 1991; 10. 100(4):1076–1081. PMID: 1914561.
15. Sengupta P, Sessler DI, Maglinger P, Wells S, Vogt A, Durrani J, et al. Endotracheal tube cuff pressure in three hospitals, and the volume required to produce an appropriate cuff pressure. BMC Anesthesiol. 2004; 11. 4(1):8. PMID: 15569386.

16. Hoffman RJ, Parwani V, Hahn IH. Experienced emergency medicine physicians cannot safely inflate or estimate endotracheal tube cuff pressure using standard techniques. Am J Emerg Med. 2006; 3. 24(2):139–143. PMID: 16490640.

17. Michlig SA. Anaesthetic staff cannot identify extremely high tracheal tube cuff pressures by palpation of the pilot balloon. Br J Anaesth. 2013; 8. 111(2):300–301. PMID: 23858076.

18. Li Bassi G, Ranzani OT, Marti JD, Giunta V, Luque N, Isetta V, et al. An in vitro study to assess determinant features associated with fluid sealing in the design of endotracheal tube cuffs and exerted tracheal pressures. Crit Care Med. 2013; 2. 41(2):518–526. PMID: 23263575.

19. Ulrich-Pur H, Hrska F, Krafft P, Friehs H, Wulkersdorfer B, Kostler WJ, et al. Comparison of mucosal pressures induced by cuffs of different airway devices. Anesthesiology. 2006; 5. 104(5):933–938. PMID: 16645443.

20. Horisberger T, Gerber S, Bernet V, Weiss M. Measurement of tracheal wall pressure: a comparison of three different in vitro techniques. Anaesthesia. 2008; 4. 63(4):418–422. PMID: 18336493.

21. Striebel HW, Pinkwart LU, Karavias T. Tracheal rupture caused by overinflation of endotracheal tube cuff. Anaesthesist. 1995; 3. 44(3):186–188. PMID: 7762778.
22. Seidl RO, Todt I, Nielitz T, Ernst A. Tracheal ruptures in endotracheal intubation: diagnosis and therapy. HNO. 2002; 2. 50(2):134–138. PMID: 12080623.
23. Stannard K, Wells J, Cokis C. Tracheal rupture following endotracheal intubation. Anaesth Intensive Care. 2003; 10. 31(5):588–591. PMID: 14601288.

24. Coordes A, Rademacher G, Knopke S, Todt I, Ernst A, Estel B, et al. Selection and placement of oral ventilation tubes based on tracheal morphometry. Laryngoscope. 2011; 6. 121(6):1225–1230. PMID: 21557233.

25. Dullenkopf A, Gerber AC, Weiss M. Nitrous oxide diffusion into tracheal tube cuffs: comparison of five different tracheal tube cuffs. Acta Anaesthesiol Scand. 2004; 10. 48(9):1180–1184. PMID: 15352966.

26. Chadha NK, Gordin A, Luginbuehl I, Patterson G, Campisi P, Taylor G, et al. Automated cuff pressure modulation: a novel device to reduce endotracheal tube injury. Arch Otolaryngol Head Neck Surg. 2011; 1. 137(1):30–34. PMID: 21242543.
27. Massard G, Rouge C, Dabbagh A, Kessler R, Hentz JG, Roeslin N, et al. Tracheobronchial lacerations after intubation and tracheostomy. Ann Thorac Surg. 1996; 5. 61(5):1483–1487. PMID: 8633963.

28. Lampl L. Tracheobronchial injuries: conservative treatment. Interact Cardiovasc Thorac Surg. 2004; 6. 3(2):401–405. PMID: 17670273.

29. Gordin A, Chadha NK, Campisi P, Luginbuehl I, Taylor G, Forte V. An animal model for endotracheal tube-related laryngeal injury using hypoxic ventilation. Otolaryngol Head Neck Surg. 2011; 2. 144(2):247–251. PMID: 21493425.

30. Foroulis CN, Simeoforidou M, Michaloudis D, Hatzitheofilou K. Pericardial patch repair of an extensive longitudinal iatrogenic rupture of the intrathoracic membranous trachea. Interact Cardiovasc Thorac Surg. 2003; 12. 2(4):595–597. PMID: 17670132.

Fig. 1
Experimental setup of the artificial ventilation system. The ventilation system is connected with the trachea and main bronchi via an tracheal tube placed subglottically and with the artificial lung.
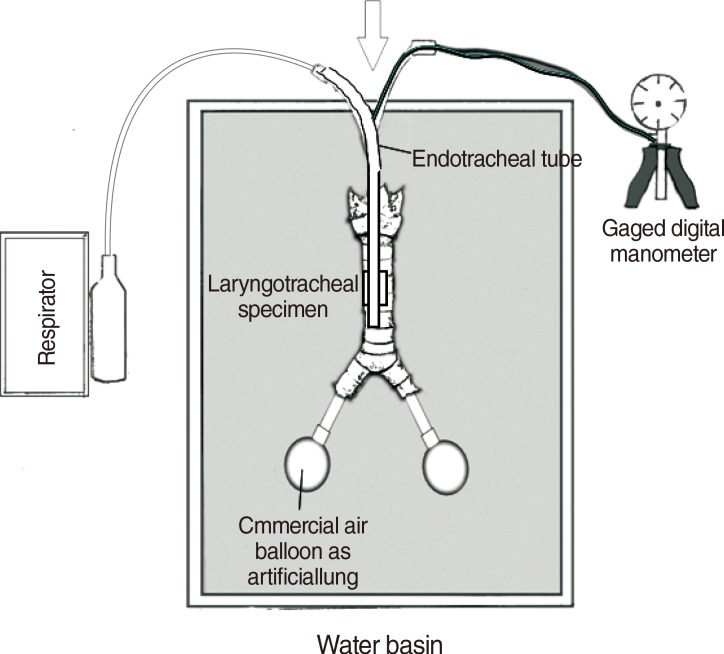
Fig. 2
Pressure rupture resistance during tracheal ruptures. (A) Pressure rupture resistance during tracheal ruptures of all investigated tracheal specimens (see Table 1) represented using a scatter diagram. (B) Relative increase in the coronal tracheal diameter with increasing pressure rupture resistance.
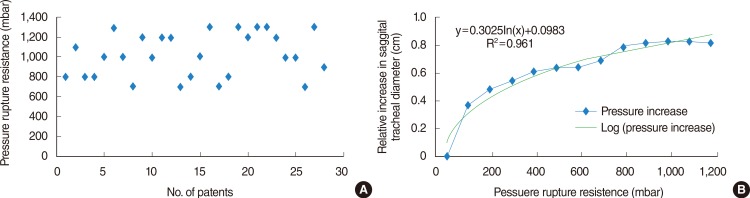
Fig. 3
Pressure rupture resistance according to suitable versus unsuitable tubes. (A) Pressure rupture resistance during tracheal ruptures of investigated tracheal specimens according to tube suitability represented using a box plot. (B) The probability of tracheal rupture is represented using the one minus probability of survival determined by Kaplan-Meier.
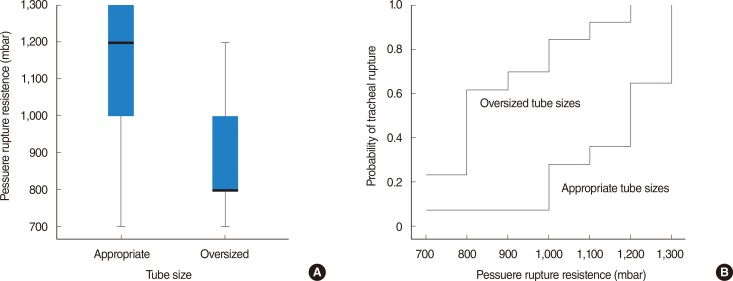
Fig. 4
Pressure rupture resistance depending on taller and shorter patients' height according to suitable (A) versus unsuitable tubes (B). Data are represented using box plots.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download