Abstract
Objectives
The aim of this study was to evaluate the component-resolved diagnosis using a microarray allergen chip (Immuno Solid-phase Allergen Chip, ImmunoCAP ISAC) and to compare this new diagnostic tool with the established ImmunoCAP methods for allergen-specific IgE detection in allergic rhinitis patients.
Methods
One hundred sixty-eight allergic rhinitis patients were included in this study. All the patients were diagnosed with allergic rhinitis according to their clinical symptoms, physical examination and a positive skin prick test. We analyzed their specific IgEs for house dust mites (Dermatophagoides farine [DF] and Dermatophagoides pteronyssinus [DP]), Alternaria alternata, birch, and mugwort using ImmunoCAP and ImmunoCAP ISAC in the same patient sample. We compared the sensitivity and correlation between the two tests.
Results
In cases of allergies to DP and DF, the sensitivity of the specific IgE was 80% and that of the allergen microarray was 78.9%. The correlation between the two tests was significant for both DP and DF (P<0.001). For the A. alternata, birch and mugwort allergens, the sensitivity of ImmunoCAP ISAC was slightly lower than that of ImmunoCAP.
Molecule-based diagnosis has improved the clinical utility of allergy tests. Recently, the component-resolved allergen microarray chip technique was introduced to the molecule-based diagnostic allergology field. In contrast to the traditional specific IgE assays, this method does not use whole extracts from allergens but instead uses multiple purified natural and recombinant allergen components spotted onto a microarray plate [1]. The older specific IgE test can detect only one allergen even though a patient may be sensitized to multiple allergens. However, the microarray technique can be used to determine specific IgEs against multiple allergens simultaneously and even allow the determination of the IgMs and IgGs at the same time. It also explains the cross reactions between allergens by confirmation of the cross-reactive allergen [2].
The Immuno Solid-phase Allergen Chip (ImmunoCAP ISAC, Phadia, Uppsala, Sweden) is a commercially available microarray-based IgE detection chip. It allows for the measurement of specific IgE antibodies to 112 allergen components of 46 major allergens in a single measurement with a minimal amount of blood or serum (30 µL) for testing, and thus this technique offers opportunities to establish individual allergy profiles [3456]. The sequential screening of the components of one or more allergens with a library has been used to diagnose several food allergies [789]. This microarray allergen chip has recently been introduced into clinical allergologic research as this tool will hopefully be useful for the measurement of serum IgEs in allergic patients with atopic dermatitis [1011].
However, there have been few studies on the clinical utility of a microarray allergen chip test for allergic rhinitis patients. Therefore, the aim of this study was to evaluate the ImmunoCAP ISAC and compare it with the established method of ImmunoCAP IgE detection.
One hundred sixty-eight allergic rhinitis patients were included in this study. All patients were adults over 18 years old. All the patients were diagnosed with allergic rhinitis by their clinical symptoms, physical examination and a positive skin prick test. The wheal reaction was greater than 3+. Ninety-five patients were allergic to house dust mites (Dermatophagoides farine [DF] and Dermatophagoides pteronyssinus [DP]), 23 were allergic to the fungus Alternaria alternata, 23 were allergic to birch, and 26 were allergic to mugwort. The mean age of the patients was 26.6±10.2 years old. The male to female ratio was 113:53. This study was approved by the Institutional Review Board of Gachon University Gil Medical Center.
A skin prick test was performed (Allergen kit, Allergopharma, Reinbek, Germany). The allergic reaction was defined as positive when the weal reaction exceeded 3+.
We analyzed the presence of specific IgEs using ImmunoCAP for house dust mites (DP and DF), A. alternata (m6), birch (Betula verrucosa) (t3), and mugwort (Artemisia vulgaris) (w6). A positive value was defined when the level of allergen-specific IgE was greater than 0.34 kU/L.
To define the allergen components, we used a microarray-based IgE detection chip, the Immuno Solid-phase Allergen Chip (ImmunoCap ISAC ver. CRD-50, VBC Genomics Bioscience Research GmbH, Vienna, Austria) according to the manufacturer's instructions [5]. This microarray chip allows the measurement of 112 allergen components of 46 allergens. Therefore, we selected the major components of house dust mites (nDer p 1, nDer p 2, nDer p 10, nDer f 1, and nDer f 2), birch (rBet v 1 and rBet v 4), mugwort (nArt v 1 and nArt v 3), and Alternaria (rAlta1 and rAlta6). A positive value was defined when the ISAC standardized units (ISU) were greater than 0.3.
The recommended method for the allergen microarray is as follows. The purified natural and recombinant allergen components were spotted on the allergen microarray chip. After incubation with 20-µL undiluted serum from allergic patients for 180 minutes at room temperature in the humid chamber, slides were rinsed and washed for 15 minutes in TBS-T (TBS-T buffer, 150 mM sodium chloride, 10 mM Tris base, and 0.5% Tween 20, pH 8.0), for 5 minutes in deionized water and dried. To detect bound IgE antibodies, allergen chips were incubated for 60 minutes at room temperature with 20 µL of a fluorescence-labelled antihuman IgE antibody. After a rinsing and washing procedure, the slides were dried completely. The slides were scanned with a laser scanner (LuxScan 10K, CapitalBio, Beijing, China). The analysis of the microarray image data was performed with ImmunoCAP ISAC Xplain (Phadia).
In cases of allergy to DP or DF, the sensitivity of the specific IgE to skin prick test was 80%, and that of the allergen microarray was 78.9%.
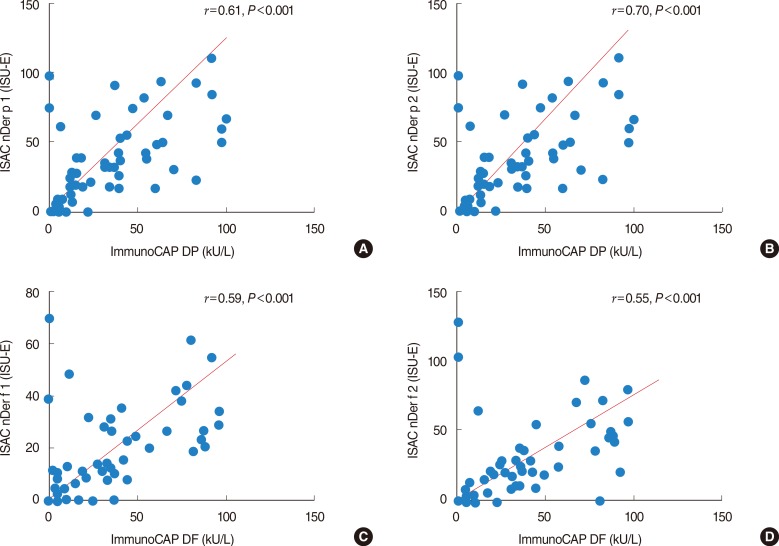
The agreement rate between the specific IgE and allergen microarray 98.6% (positive number in allergen microarray/positive number in specific IgE, 75/76). The Kappa value was 0.774 (Fig. 1A). The sample correlation coefficient for nDer p 1 was 0.61, and the P-value was <0.001 (Fig. 2A). The sample correlation coefficient for nDer p 2 was 0.70, and the P-value was <0.001 (Fig. 2B). The sample correlation coefficient for nDer f 1 was 0.59, and the P-value was <0.001 (Fig. 2C). The sample correlation coefficient for nDer f 2 was 0.55, and the P-value was <0.001 (Fig. 2D).
In cases of A. alternata (m6), the sensitivity of the specific IgE was 91.3%, and that of the allergen microarray was 76.9%. The agreement rate between the specific IgE and allergen microarray 47.6% (positive number in allergen microarray/positive number in specific IgE, 10/21). The Kappa value was 0.551 (Fig. 1B). In cases of birch (B. verrucosa) (t3), the sensitivity of the specific IgE was 86.9%, and that of the allergen microarray was 43.6%. The agreement rate between the specific IgE and allergen microarray 50% (positive number in allergen microarray/positive number in specific IgE, 10/20). The Kappa value was 0.511 (Fig. 1C). In cases of allergy to mugwort (Artemisia vulgaris) (w6), the sensitivity of the specific IgE was 92.3%, and that of the allergen microarray was 69.2%. The agreement rate between the specific IgE and allergen microarray 50% (positive number in allergen microarray/positive number in specific IgE, 18/24). The Kappa value was 0.670 (Fig. 1D).
The findings of this study demonstrate that the results of the ISAC were comparable with the results of the traditional ImmunoCap IgE detection for house dust mites. However, in cases of A. alternata, birch and mugwort, the ISAC was less sensitive than the allergen-specific IgE detection method.
Microarray testing for allergen-specific IgEs can be presumed to be the method of choice for a prospective component-resolved diagnosis of type I allergy [12]. There is still controversy about the sensitivity of this microarray method compared with traditional IgE tests. The ISAC is known to have higher variability when the serum IgE level is low. Variable results in the analysis of certain allergens and some limitations in the types of allergen sources have also been shown [13]. The microarray has a good dynamic range, similar to that of the CAP/RAST system. The microarray and UniCAP showed comparable analytical sensitivity, exceeding the skin test [12]. A comparison of these two tests revealed that they were equally relevant in the diagnosis of patients allergic to grass, birch and cats. However, the microarray is slightly less sensitive for the diagnosis of house dust mite-allergic patients and less sensitive in mugwort allergies [14]. The ISAC CRD103 and whole-allergen CAP showed similar high sensitivity and specificity in diagnosing grass and cypress pollen allergies [15]. A comparison of the ISAC with the ImmunoCAP 250 revealed that the concordance rate was 78.65% and that the concordance rate was 93.75% for negative results. No nonspecific binding was observed [16]. In the nDer p 1, nDer p 2, and nDer p 10 analyses, the ImmunoCAP and ISAC were concordant, but the quantitative correlation was poor [17]. There was no clinical implication for this difference. The reproducibility of the ISAC was good. The positive percent agreement varied between 75% and 100% for sIgE levels above 1 kUA/L [18]. Our results showed that the two tests have almost the same sensitivity for house dust mites. However, the ISAC sensitivity was very low for A. alternata, birch, and mugwort compared with the results of previous studies. We believe that the ImmunoCAP has included all the components of the allergen extracts; however, the ISAC has included some limited types of components from one allergen source. These allergen components might suggest a lower sensitivity in the ISAC compared with the ImmunoCAP.
In the ImmunoCAP ISAC tests, the IgE antibody levels are reported in 4 levels using the ISU-E, which contrasts with the kU/L measurements in the ImmunoCAP with 6 classes. This difference in the measurement units with the same IgE levels might influence the interpretation of a positive level between the two tests. A component-resolved allergy diagnosis with recombinant allergens reveals that the IgE reactivity profiles to individual birch pollen allergens vary among European populations [19]. Our results might distinguish the characteristics of the Korean people with this microarray chip test.
Recently, the WAO-ARIA-GALEN consensus document on molecule-based allergy (MA) diagnostics provided a practical guideline for MA diagnostics. One of the most important implications of an MA diagnosis is its ability to distinguish genuine sensitization from sensitization due to cross-reactivity [13].
In contrast, a microarray allergen chip can be the parameter that decides whether to start immunotherapy or to monitor the effects of immunotherapy. An MA diagnosis distinguishes the major allergens between ragweed and mugwort pollen allergies and has also shown extensive cross-reactivity for both groups. Therefore, it provides the major allergen information for the appropriate immunotherapy [20]. Some authors have reported that immunotherapy for house dust mite allergies seems less efficient than immunotherapy with pollen allergens and that the treatment for house dust mite allergies has a high rate of side-effects [2122]. The microarray allergen chip includes purified major allergens (i.e., nDer p 1, nDer p 2, nDer f 1, and nDer f 2), which are major components of the immunotherapy therapeutic allergen extracts [2324]. Therefore, using the microarray allergen chip, a clinician can identify the patients suitable for immunotherapy with a nDer p/nDer f extract. In cases of patients with broad-reactive components such as nDer p 10, the immunotherapy for house dust mite may have less effect on those patients [24]. In addition, the microarray allergen chip can be used to monitor the effects of immunotherapy. With a minimal amount of blood or serum, the changing levels of allergen-specific IgGs after immunotherapy can be evaluated. However, further large-scale studies are needed before the allergen microarray can be used in daily clinical practice.
In conclusion, the results of this study suggest that the allergen microarray is a reliable method to diagnose allergic rhinitis to DP and DF. Further study on the utility of the allergen microarray is needed.
References
1. Harwanegg C, Laffer S, Hiller R, Mueller MW, Kraft D, Spitzauer S, et al. Microarrayed recombinant allergens for diagnosis of allergy. Clin Exp Allergy. 2003; 1. 33(1):7–13. PMID: 12534543.

2. Ferrer M, Sanz ML, Sastre J, Bartra J, del Cuvillo A, Montoro J, et al. Molecular diagnosis in allergology: application of the microarray technique. J Investig Allergol Clin Immunol. 2009; 19(Suppl 1):19–24.
3. Mothes N, Valenta R, Spitzauer S. Allergy testing: the role of recombinant allergens. Clin Chem Lab Med. 2006; 44(2):125–132. PMID: 16475895.

4. Lidholm J, Ballmer-Weber BK, Mari A, Vieths S. Component-resolved diagnostics in food allergy. Curr Opin Allergy Clin Immunol. 2006; 6. 6(3):234–240. PMID: 16670520.

5. Ebo DG, Bridts CH, Verweij MM, De Knop KJ, Hagendorens MM, De Clerck LS, et al. Sensitization profiles in birch pollen-allergic patients with and without oral allergy syndrome to apple: lessons from multiplexed component-resolved allergy diagnosis. Clin Exp Allergy. 2010; 2. 40(2):339–347. PMID: 19709127.

6. Shreffler WG. Microarrayed recombinant allergens for diagnostic testing. J Allergy Clin Immunol. 2011; 4. 127(4):843–849. PMID: 21458654.

7. Vereda A, Andreae DA, Lin J, Shreffler WG, Ibanez MD, Cuesta-Herranz J, et al. Identification of IgE sequential epitopes of lentil (Len c 1) by means of peptide microarray immunoassay. J Allergy Clin Immunol. 2010; 9. 126(3):596–601.e1. PMID: 20816193.

8. Cerecedo I, Zamora J, Shreffler WG, Lin J, Bardina L, Dieguez MC, et al. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J Allergy Clin Immunol. 2008; 9. 122(3):589–594. PMID: 18774394.

9. Ott H, Baron JM, Heise R, Ocklenburg C, Stanzel S, Merk HF, et al. Clinical usefulness of microarray-based IgE detection in children with suspected food allergy. Allergy. 2008; 11. 63(11):1521–1528. PMID: 18925888.

10. Lin J, Bardina L, Shreffler WG. Microarrayed allergen molecules for diagnostics of allergy. Methods Mol Biol. 2009; 2. 524:259–272. PMID: 19377951.

11. Wöhrl S. The potential of allergen biochips. Recent Pat Inflamm Allergy Drug Discov. 2008; 11. 2(3):186–190. PMID: 19076008.
12. Jahn-Schmid B, Harwanegg C, Hiller R, Bohle B, Ebner C, Scheiner O, et al. Allergen microarray: comparison of microarray using recombinant allergens with conventional diagnostic methods to detect allergen-specific serum immunoglobulin E. Clin Exp Allergy. 2003; 10. 33(10):1443–1449. PMID: 14519153.

13. Canonica GW, Ansotegui IJ, Pawankar R, Schmid-Grendelmeier P, van Hage M, Baena-Cagnani CE, et al. A WAO - ARIA - GA2LEN consensus document on molecular-based allergy diagnostics. World Allergy Organ J. 2013; 10. 6(1):17. PMID: 24090398.
14. Wohrl S, Vigl K, Zehetmayer S, Hiller R, Jarisch R, Prinz M, et al. The performance of a component-based allergen-microarray in clinical practice. Allergy. 2006; 5. 61(5):633–639. PMID: 16629796.

15. Cabrera-Freitag P, Goikoetxea MJ, Beorlegui C, Gamboa P, Gastaminza G, Fernandez-Benitez M, et al. Can component-based microarray replace fluorescent enzimoimmunoassay in the diagnosis of grass and cypress pollen allergy? Clin Exp Allergy. 2011; 10. 41(10):1440–1446. PMID: 21749500.

16. Gadisseur R, Chapelle JP, Cavalier E. A new tool in the field of in-vitro diagnosis of allergy: preliminary results in the comparison of ImmunoCAP© 250 with the ImmunoCAP© ISAC. Clin Chem Lab Med. 2011; 2. 49(2):277–280. PMID: 21143018.

17. Bronnert M, Mancini J, Birnbaum J, Agabriel C, Liabeuf V, Porri F, et al. Component-resolved diagnosis with commercially available D. pteronyssinus Der p 1, Der p 2 and Der p 10: relevant markers for house dust mite allergy. Clin Exp Allergy. 2012; 9. 42(9):1406–1415. PMID: 22747483.
18. Wulfert F, Sanyasi G, Tongen L, Watanabe LA, Wang X, Renault NK, et al. Prediction of tolerance in children with IgE mediated cow's milk allergy by microarray profiling and chemometric approach. J Immunol Methods. 2012; 8. 382(1-2):48–57. PMID: 22580759.

19. Movérare R, Westritschnig K, Svensson M, Hayek B, Bende M, Pauli G, et al. Different IgE reactivity profiles in birch pollen-sensitive patients from six European populations revealed by recombinant allergens: an imprint of local sensitization. Int Arch Allergy Immunol. 2002; 8. 128(4):325–335. PMID: 12218371.

20. Gadermaier G, Wopfner N, Wallner M, Egger M, Didierlaurent A, Regl G, et al. Array-based profiling of ragweed and mugwort pollen allergens. Allergy. 2008; 11. 63(11):1543–1549. PMID: 18925891.

21. Bousquet J, Michel FB. Specific immunotherapy in asthma. Allergy Proc. 1994; Nov-Dec. 15(6):329–333. PMID: 7721083.

22. Mellerup MT, Hahn GW, Poulsen LK, Malling H. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000; 10. 30(10):1423–1429. PMID: 10998019.

23. Meyer CH, Bond JF, Chen MS, Kasaian MT. Comparison of the levels of the major allergens Der p I and Der p II in standardized extracts of the house dust mite, Dermatophagoides pteronyssinus. Clin Exp Allergy. 1994; 11. 24(11):1041–1048. PMID: 7874602.
24. Pittner G, Vrtala S, Thomas WR, Weghofer M, Kundi M, Horak F, et al. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004; 4. 34(4):597–603. PMID: 15080813.

Fig. 1
The comparison of sensitivity. (A) The specific IgE and allergen-microarray to Dermatophagoides farine and Dermatophagoides pteronyssinus. The sensitivity of the specific IgE was 80%, and that of the allergen-microarray was 78.9%. The Kappa value was 0.774. (B) The specific IgE and allergen microarray to fungus. The sensitivity of the specific IgE was 91.3%, and that of the allergen microarray was 76.9%. The Kappa value was 0.551. (C) The specific IgE and allergen microarray to tree pollen. The sensitivity of the specific IgE was 86.9%, and that of the allergen microarray was 43.6%. The Kappa value was 0.511. (D) The specific IgE and allergen microarray to weed pollen. The sensitivity of the specific IgE was 92.3%, and that of the allergen-microarray was 69.2%. The Kappa value was 0.670.

Fig. 2
The correlation of specific IgE vs. ImmunoCAP ISAC. (A) The specific IgE to Dermatophagoides pteronyssinus (DP) and nDer p 1. The correlation coefficient for nDer p 1 was 0.61, and the P-value was <0.001. (B) The specific IgE to DP and nDer p 2. The correlation coefficient for nDer p 2 was 0.70, and the P-value was <0.001. (C) The specific IgE to Dermatophagoides farine (DF) and nDer f 1. The correlation coefficient for nDer f 1 was 0.59, and the P-value was <0.001. (D) The specific IgE to DF and nDer f 2. The correlation coefficient for nDer f 2 was 0.55, and the P-value was <0.001. ISU-E, ISAC standardized unit.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download