INTRODUCTION
Neuroblastoma (NBL), a malignant embryonic tumor of the neural crest cells, is the most common tumor in children less than 1 year old and predominantly affects children under 5 years of age [
12]. NBL may arise anywhere along the sympathetic nervous system, but are found most frequently in the adrenal glands or elsewhere in the abdomen, chest, or pelvis [
1]. Treatment is based on the risk stratification using age, stage,
MYCN oncogene status and pathologic classification [
2]. Nowadays, patients with high-risk NBL are treated with dose-intensive chemotherapy, which typically includes cisplatin or a myeloablative dose of carboplatin, in order to maximize survival [
3]. However, this intensive chemotherapy can increase the risk of ototoxicity, because cisplatin or carboplatin are well-known ototoxic agents [
23]. Platinum-related sensorineural hearing loss is generally irreversible, and occurs bilaterally in 22%-70% of children [
23]. The impact of hearing loss in patients with NBL is particularly significant because nearly 90% of patients are less than 5 years old when they are exposed to the agents [
1].
Thus, early detection of hearing loss and proper intervention/rehabilitation is essential [
3]. Although most patients with hearing loss due to ototoxic agents can be rehabilitated with hearing aids, amplification by hearing aids is usually not sufficient for the patients with profound hearing loss. In the specific population with profound hearing loss, cochlear implantation (CI) is mandatory for proper hearing rehabilitation. As the platinum-induced hearing loss progresses from high to low frequencies, residual low-frequency hearing is typically observed, even in candidates for CI.
During CI, electrodes are inserted into the cochlea through the round window or cochleostomy site, and subsequent hearing loss can occur due to a leak of perilymph, disruption of the basilar membrane, fractures of the osseous spiral lamina, or tearing of the endosteum of the scala tympani [
4]. The deterioration in hearing also could be attributable to a disturbance in cochlear mechanics. The impedance of the stapes movements may be increased by postimplantation intrascalar fibrosis [
5]. However, the importance of residual low-frequency hearing has increased because of recent evidence that residual hearing is essential in increasing hearing performance in noisy listening environments, improving music perception, and giving sound a more natural quality [
4]. Thus, surgical techniques have been meticulously developed to improve the chance of preserving residual hearing. 'Soft surgery' techniques involving a shorter insertion depth, off-stylet technique, and changes in the angle of insertion have been proposed and used [
4]. The 'soft surgery' technique allows for the preservation of residual low-frequency hearing and the subsequent application of 'electro-acoustic' stimulation (EAS), while the lower frequencies are aided through acoustic amplification and the mid/high frequencies are aided through the cochlear implant in the same ear [
4]. Though patients who underwent CI after treatment of NBL can be good candidates for EAS, there have been no reports of CI using the hearing preservation technique in NBL patients. Thus, the aims of this study are to evaluate speech performance and hearing preservation of CIs in patients with NBL who developed severe hearing loss following multimodal therapies.
Go to :

MATERIALS AND METHODS
Study design
A retrospective medical chart review was performed in CI recipients from December 2010 to January 2014, and three patients with a history of NBL were identified. Detailed medical records were reviewed for oncologic treatment modality, pre- and postoperative audiometry, the perioperative surgical course, and the postoperative speech performance outcome.
Treatment modality
Treatment modalities for NBL in our hospital are composed of chemotherapy, radiation therapy, surgical excision, and peripheral blood stem cell transplantation (PBSCT). All patients were treated with multiple cycles of induction chemotherapy, including cisplatin or carboplatin. Based on the treatment response of the tumor after induction chemotherapy, additional therapeutic methods were combined. That is, patient 1 underwent additional chemotherapy for a suspicious relapse of NBL. Patient 2 underwent excision surgery and PBSCT, and patient 3 was treated with additional chemotherapy and whole-body irradiation.
Hearing evaluation
Hearing evaluation was routinely conducted in patients who underwent chemotherapy before and after the treatment. Play (behavioral) audiometry was performed in patients whose age was more than 3 years. Auditory brainstem response (ABR) was substituted for play audiometry if the patient could not complete the play audiometry. Hearing aids were applied when the pure tone average (PTA; 0.5, 1, and 2 kHz) in the both ears was above 40 dB. As cisplatin-induced ototoxic effects can develop several years after treatment, regular audiometric follow-up was recommended.
Decision of CI
The determination that the subjects were candidates for CI was reached through a variety of evaluations, including audiologic evaluations, including PTA or play audiometry, ABR, otoacoustic emission, and auditory steady state response. The decision to undergo CI was based on the hearing level as well as a determination of no significant benefit from the use of hearing aids. The criteria for CI were as follows: (1) >70 dB of PTA or play-audiometry threshold at 0.5, 1, and 2 kHz who completed behavioral audiometry, or >90 dB ABR threshold who failed behavioral audiometry in both ears; and (2) no benefits from use of hearing aids. The criteria for EAS were PTA threshold below 65 dB at frequencies lower than 750 Hz.
Postoperative follow-up and evaluation of residual hearing
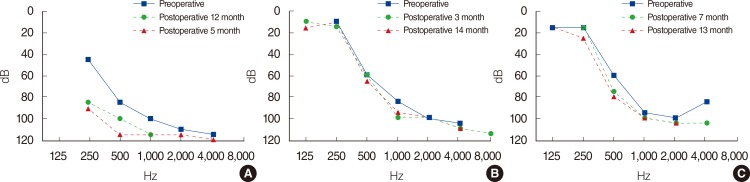
Speech performance and hearing preservation were evaluated using a postoperative speech tests and PTA. Hearing preservation was calculated by examining the changes in the average hearing threshold at 250, 500, and 1,000 Hz between the preoperative and postoperative measures. To categorize the change of hearing threshold, the following scale was used: a ≤10 dB change, complete hearing preservation; 11-20 dB change, moderate preservation; 21-40 dB, marginal preservation; and >40 dB or scaled out, no hearing preservation [
4]. Hearing and speech evaluations were performed preoperatively and 12 months after CI. Speech evaluation included the category of auditory performance (CAP) score, and mono-/bi-syllable and sentence identification tests.
Go to :

DISCUSSION
NBL is the most common extracranial solid tumor in children and accounts for 7% of all childhood cancers [
6]. NBL accounting for about 27% in Europe and 26% in United States infants of all malignancies diagnosed [
78]. Though the prognosis of low- and intermediate-risk patients with NBL is excellent, high-risk patients had a long-term survival rate of less than 15% before the 1990s [
69]. However, the use of aggressive multimodal therapies have resulted in better outcomes, with an event-free survival rate 3 years after treatment of 40%-60% [
2]. A large proportion (up to 90%) of survivors of the high-risk NBL has been reported to experience long-term side effects [
6]. The most prominent long-term toxicities found in NBL survivors include hearing loss, renal toxicity, gonadal insufficiency, hypothyroidism, growth failure, and secondary malignancies [
6].
Hearing loss is one important potential treatment-related late effect, usually caused by platinum-containing chemotherapies, particularly cisplatin and carboplatin. Platinum agents are effective in the treatment of a variety of malignancies in adults and children [
10]. A high cumulative dose of platinum chemotherapy is included in multimodal treatment protocols for intermediate- and high-risk NBL [
11]. Platinum-related ototoxicity can lead to the destruction of cochlear sensory hair cells, in both the high (>2,000 Hz) and lower frequency ranges [
11]. Though the amount of these ototoxicity can be influenced by age, concurrent medications, renal function, and cranial irradiation [
12], cumulative doses of increased platinum chemotherapy (400 mg/m
2) may be the main determining factor [
1113]. Platinum-induced ototoxicity is typically characterized by irreversible, bilateral, high-frequency hearing loss [
14]. The long-term negative consequences of cisplatin-induced hearing loss include poor speech/language development and subsequent poor academic and social outcomes later in life [
15]. As treatment-related ototoxicity can progress several years after the completion of treatment, regular auditory monitoring is mandatory because the early detection of hearing loss is important. Previous studies have suggested ototoxicity grading criteria for the detection and intervention for hearing loss after multimodal treatment in NBL patients.
Even minimal-to-mild hearing loss can significantly impact language development, verbal abilities, and reasoning skills in young children [
11]. This is of particular concern in children with NBL who receive platinum-based chemotherapy, because they are often still in the process of learning language [
11]. In fact, it has been shown in several studies that children with even mild bilateral or unilateral hearing loss have more difficulties with language acquisition and often score more poorly in vocabulary and spelling compared with normal hearing children [
16]. Concerns about aggravation of hearing loss in patients treated with platinum-based chemotherapy have been raised because long-term ototoxic effect of chemotherapy had been reported [
12]. It typically starts with high frequencies (4,000-8,000 Hz), and progresses to the speech frequencies (500-2,000 Hz) with increasing cumulative exposure [
317]. Physicians should pay attention to these specific populations who have undergone platinum-based chemotherapy, because insufficient hearing amplification can affect speech and language acquisition, academic achievement, and psychosocial development [
13]. Hearing loss by treatment-related ototoxicity often requires devices for assistance. Hearing amplification strategies are usually recommended, such as hearing aids, in children whose hearing thresholds are greater than 40 dB [
18]. Furthermore, hearing loss can be progressive, even after completion of the treatment [
12].
There is a report about CI after combination therapy for medulloblastoma [
14]. However, platinum-base chemotherapies are still actively used for a variety of childhood malignancies including medulloblastoma, NBL, osteosarcoma, hepatoblastoma, germ cell tumors, and certain brain tumor [
13]. Consequently, profound hearing loss which requires cochlear implants can be developed in children with malignancies other than medulloblastoma. If patients with severe to profound hearing loss experience a limited benefit from conventional hearing amplification using hearing aids, CI should be considered as the next hearing rehabilitation strategy to prevent further disadvantages from hearing loss.
EAS is based on the concept of preserving residual low-frequency acoustic hearing in the implanted ear, and its combination with electrical stimulation via the CI is used to improve high frequency hearing loss [
19]. Residual hearing in CI implantees have been shown to increase hearing environments, improve music perception and appreciation, and give sound and voices a more natural quality [
4]. As a variety of benefits have been reported by using EAS, hearing preservation should be an important goal during CI in patients with NBL.
In this study, we analyzed 3 patients who had undertaken CI for profound bilateral hearing loss after treatment of NBL. As stated earlier, platinum-based chemotherapy typically leads to high-frequency hearing loss. Thus, low-frequency hearing (especially 250, 500 Hz) may be preserved in these patients. It means they can be good candidates for EAS. All three patients in this study had residual hearing at frequencies <1,000 Hz, though the average hearing threshold (500, 1,000, and 2,000 Hz) was >70 dB. Thus, to preserve residual low-frequency hearing, a soft surgery technique was applied to all patients along with a short-length electrode (Flex EAS) in patients 2 and 3. Though patient 1 experienced some hearing deterioration postoperatively, partial hearing preservation was achieved. Complete hearing preservation was achieved in patients 2 and 3 one year after CI.
The limitation of this study is its retrospective design. Regular and unified auditory monitoring is needed for the early detection of treatment-related hearing loss and rapid hearing rehabilitation. It may indicate that hearing preservation can be achieved in hearing loss from NBL treatment, and surgeons should try to use a soft surgical technique in order to preserve residual hearing during CI.
In conclusion, a sloping-type of hearing loss can develop in patients with NBL who underwent multimodal therapy. EAS can be a good treatment option for this specific population, and this study showed the possibility and benefits of hearing preservation in these patients by using a soft surgery technique during CI surgery.
Go to :





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download