DISCUSSION
The prevalence of aural atresia or stenosis varies from 55% to 93% [
4]. Generally, congenitally malformed canals can be classified into bony stenosis, membranous atresia and bony atresia. In the membranous type, there is a soft tissue plug at the position of the tympanic membrane, whereas bony stenosis is characterized by the presence of a bony plate at the level of the tympanic membrane [
5]. This is the first report to compare the three groups' preoperative temporal CT imaging. Our results indicated that there indeed extensive anatomical variations between the three groups, and we discovered some results that contradict existing knowledge.
Microtia is usually accompanied by atresia or stenosis of the EAC. For the degree of microtia, various classifications have been made according to the severity of the existing malformation. The first classification was created by Marx in 1926. Different classification systems were described later by Jahrsdoerfer and Aguilar (1988), Altman (1955), Lapchenko (1967), Gill (1969), Rogers (1974), Tanzer (1977), and Tasse (2005) [
6]. We used Marx grading to assess the auricle development in patients with congenital malformed canals. The study indicated the auricular development mainly manifested as degree III, II and normal in the atresia group, the stenosis group and the normal group, respectively (
P<0.001) (
Table 2). In addition, 86% (31 of 35) of ears with major microtia (third-degree dysplasia) had an atresia; in 54.8% (23 of 42) of the minor microtic (first-degree or second-degree) ears, the bony or cartilaginous part of the EAC was stenotic (
Fig. 8). Compared with CAA ears, the auricle development was better in EACS ears. This finding can help otologists to predict the EAC development status from the auricle appearance in an outpatient before physical examination and further CT inspection.
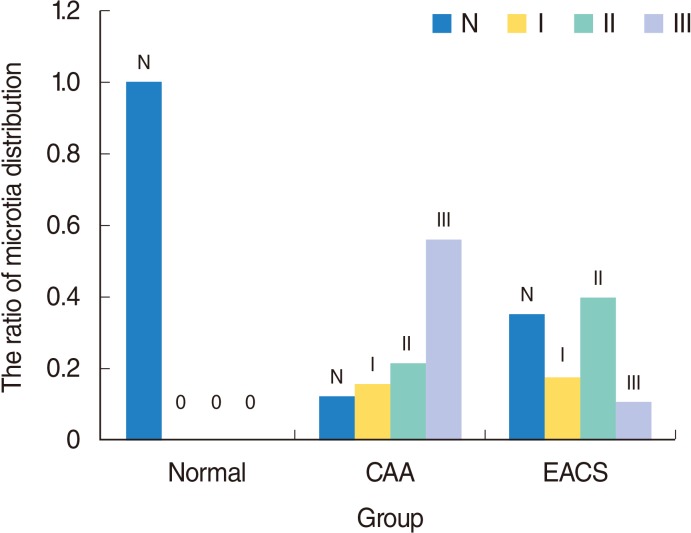 | Fig. 8The microtia distribution in Marx classification in the three groups. N represent normal auricle. Grades I, II, and III represent different deformity of auricle in Marx classification. CAA, congenital aural atresia; EACS, external auditory canal stenosis.
|
Table 2.
The microtia distribution in Marx classification in the three groups
|
Group |
Grade
|
Total |
|
I |
II |
III |
Normal |
|
CAA |
8 (14.5) |
11 (20.0) |
31 (56.4) |
5 (9.1) |
55 |
|
EACS |
7 (17.1) |
16 (39.0) |
4 (9.8) |
14 (34.1) |
41 |
|
Normal |
0 |
0 |
0 |
46 (100) |
46 |
|
Total |
15 (10.6) |
27 (19.0) |
35 (24.6) |
65 (45.8) |
142 |

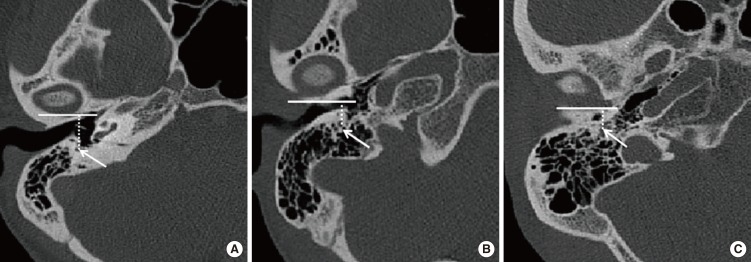
With external auditory canal atresia or stenosis, adjunct skull base structures tend to migrate toward to the area ordinarily occupied by the external canal. The TMJ is higher than usual and posteriorly displaced. The distance between the mastoid facial nerve and the TMJ is shortened [
7]. Surgery for congenital CAA/EACS is one of the most challenging and difficult procedures in otology because this condition is often accompanied by various temporal bone anomalies, such as an aberrant FN [
8910]. In aural atresia, the second genu is often located more anteriorly than usual, and the descending mastoid FN will frequently curve anteriorly toward the area of the TMJ [
11], occasionally laterally displacing the lateral wall of the tympanic cavity (
Fig. 5) [
12]. Through statistical analysis, we see that for patients with CAA/EACS, the VFN starting point and ending point was more anteriorly displaced than in those with a normal canal (
P<0.05). We also observed that the VFN is more anteriorly displaced in starting point and ending point in patients with CAA than in those with EACS, but this difference was not significant (
P>0.05). Therefore, there is possibly a shorter distance to reach the facial canal in deformed ears during a canaloplasty or tympanoplasty compared with normal ears, and theoretically, the probability of an FN injury may be more and the operation may take more dangerous.
The medial canal diameter [
13] is the distance from the VFN to the bony posterior wall of TMJ. The dimension is the shortest distance from the FN to the most posterior aspect of the TMJ and is an indicator of the medial diameter of the EAC, or its potential diameter in atretic and stenosis ears. It incorporates the key surgical anterior boundary, the TMJ, and posterior boundary, the FN. The average medial canal diameter was 6.98 mm in the atretic group, 9.18 mm in the stenosis group and 11.23 mm in the normal group (
P<0.05). Jahrsdoerfer et al. [
14] reported the average distance between the mandibular condyle and the anterior face of the mastoid bone was 11.58 mm on the atretic side, 13.02 mm on the stenosis side and 18.36 mm on the normal side. Lumbroso et al. [
9] reported that 16% of temporal mandibular joints had posterior positions in sixty-seven cases of CAA/EACS. For patients with CAA/EACS, the temporal mandibular joint backward-shift is directly caused by abnormal EAC formation due to the temporal tympanic portion hypoplasia. TMJ backward-shift, together with VFN anterior displacement, leads to medial canal diameter that is smaller in patients with CAA than those with EACS. The distance from the VFN to the bony posterior wall of the TMJ is a safe surgical margin for canaloplasty; hence, there will be a much larger operation space in EACS patients than in those with CAA. Considered with the above finding that the VFN position was not statistically significant (
P>0.05) between the CAA and EACS, we speculate that the TMJ backward-shift contributed more to the reduction of the medial canal diameter.
Whether for the general otitis media surgery or ear canaloplasty, the tegmen mastoideum is the safe operative superior margin. If the tegmen mastoideum is in a relatively inferior position, even associated with the low-lying middle cranial fossa [
15], it may limit access to the attic and middle ear during a lateral approach; this may preclude atresiaplasty, or possibly make atresiaplasty difficult because the otologist tends to work parallel to the tegmen mastoideum during atresiaplasty [
16]. Dedhia et al. [
17] reported a qualitative anatomic analysis of 130 CT scans (98 children, 32 bilateral) of CAA/EACS; approximately 13% of the ears had mild inferior displacement of tegmen, and 4% had a significantly obstructing tegmen. We quantitatively analyzed the position change of the tegmen and its relationship with the LSCC in different developmental canals. A low-lying position of the tegmen seemed more prevalent in atretic group than in the stenosis and normal groups in clinical practice. However, we did not detect any significant differences between CAA ears and EACS ears, or CAA/EACS ears and normal ears (
P>0.05) (
Figs. 9,
10). This may be a false appearance due to the hypoplastic nature of the tympanic cavity and mastoid aeration in such patients. The finding implies there may be a low correlation between the tegmen and EAC development. Therefore, we can see the safe operative superior margin is not different in a lateral approach and its position difference can be ignored in cases of atresia, stenosis and normal canal patients.
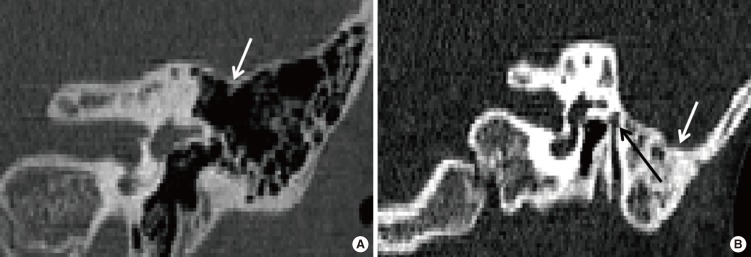
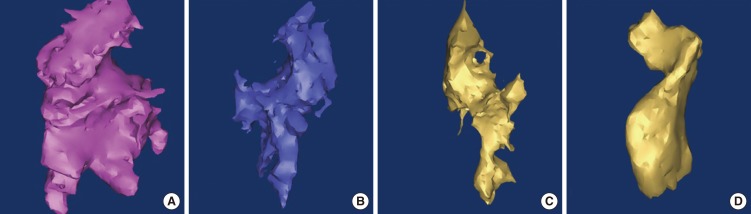 | Fig. 9Three-dimensional reconstruction tympanic cavity in left normal ear (A), in left stenosis ear (B), in left atresia ear (C, D).
|
 | Fig. 10Comparison results of different parameters. Data are the mean±SD. MCD, medial canal diameter; VFN-FS, distance from vertical facial nerve (VFN) to the coronal plane through the middle point of foramen spinosum; LSCC-TM, distance from the middle point of lateral semicircular canal caliber to the tangent through the lowest point of tegmen mastoideum; TCV, tympanic cavity volume; MIC, malleus-incus complex; CAA, congenital aural atresia; EACS, external auditory canal stenosis. *P<0.05, statistical significance in pair-wise comparison. †The starting point of VFN. ‡The ending point of VFN.
|
The size of the tympanic cavity is extremely important for tympanoplasty. First, underdevelopment of the tympanic cavity will pose additional difficulty [
10] to the otologist by limiting the space of the surgical field [
16]. Second, the tympanic cavity has a close relationship with hearing [
13]; the lack of tympanic cavity aeration can be a sole anatomical factor predictive of a poor audiometric outcome [
18]. Considering these 2 factors, we chose to measure the tympanic cavity volume. At present, there are two methods used in evaluating the tympanic cavity aeration. One method is measuring the width of the tympanic cavity, which is simple but not accurate [
192021]. The other method is measuring the volume using three dimensional reconstructions [
192021], which is more accurate. We chose the second method. The mean tympanic cavity volume was 0.36 cm
3 in the atresia group, 0.40 cm
3 in the stenosis group and 0.57 cm
3 in the normal group (
P<0.05). Ikui et al. [
19] estimated normal tympanic cavity volumes to be 0.45 cm
3 in infants and 0.61 cm
3 in adults. Osborn et al. [
22] reported the average tympanic cavity volume of the atretic ears (mean age, 4.7 years) was 0.34 cm
3 compared to an average of 0.51 cm
3 for the nonatretic ears. This result implies that the EACS ears have a more well pneumatized tympanic cavity than CAA ears, which supports our surgical outcome that EACS patients have better postoperative hearing improvement than CAA patients [
22]. By considering the development of EAC status and tympanic cavity volume, the otologist may be able to better assess surgical candidacy.
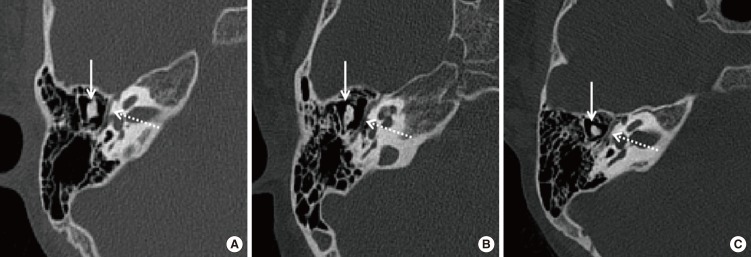
Except for the stapes, the ossicular chain develops from Meckel's cartilage and Reichert's cartilage simultaneously, and the incus and malleus are generally involved together in aural atresia cases [
10]. In CAA/EACS ears, ossicle anomalies include malleus-incus fusion [
9] and partial or total absence of ossicles [
2324]. In this study, MIC or MIJ area was measured in two dimensions to indicate the development status of ossicles. We did not measure the area of the stapes, for its display is not clear, but we confirmed its status in surgery for surgical patients. The average MIC/MIJ area was 15.63 mm
2 in the atresia group, compared to 20.20 mm
2 in the stenosis group and 21.54 mm
2 in the normal group (
P<0.001). The measurement demonstrates that MIC/MIJ development in EACS patients is better than in CAA patients, and in EACS/CAA patients the MIC/MIJ development is worse than in those with normal ears. MIC/MIJ hypoplasia or fusion obstructs the sound conduction, which leads to different degrees of hearing loss in CAA/EACS [
25]. The incus and malleus were underdeveloped, and these have to be considered during reconstructive surgery, such as partial ossicular replacement prosthesis.
Based on the above discussion, we can see the significant temporal structural differences between the CAA and EACS groups. Congenital EACS, as a special canal status, should not merely be regarded as incomplete atresia but should be regarded as an independent disease. In addition, we find that there were no significant differences between CAA ears and EACS ears (P>0.05) for the position of the tegmen mastoideum and the VFN. We were not able to demonstrate correlations between anatomic findings and hearing outcomes because of the small number of atresiaplasty cases, variable times of audiologic testing, and missing data. More research needs to be performed in the future. Therefore, the study should be interpreted as purely anatomic, with the finding of the dramatic anatomic differences for the auricle, canal, FN, tympanic cavity and MIC in patients with CAA, EACS, and normal canals.
In conclusion, the mal-development of external auditory canal is significantly associated with the auricle, middle ear developmental anomalies. Compared with CAA ears, EACS have better development of the auricle, canal, tympanic cavity and MIC and relatively safer surgical operation except for the position of the tegmen mastoideum and the VFN.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download