Abstract
Objectives
In this study we investigated the probable protective effects of thymoquinone on amikacin-induced ototoxicity in rats.
Methods
Thirty-two healthy rats were divided into four groups (amikacin, amikacin+thymoquinone, thymoquinone, and no treatment). Thymoquinone was fed to the rats via oral gavage in a dose of 40 mg/kg/day throughout the study period of 14 days. Amikacin was given by the intramuscular route in a dose of 600 mg/kg/day. Audiological assessment was conducted by the distortion product otoacoustic emission (DPOAE) and auditory brainstem response (ABR) tests, administered to all rats at the beginning of the study, and also on days 7 and 15. Biochemical parameters were calculated at the termination of the study to evaluate the oxidative status.
Results
There were significant decreases in DPOAE values and significant increases in ABR thresholds of the amikacin group on days 7 and 15, as compared to the amikacin+thymoquinone group. While ABR thresholds of the amikacin group increased significantly on days 7 and 15 as compared to their initial values, there were no significant differences between the initial and the 7th and 15th day values of ABR thresholds in the amikacin+thymoquinone group. Total oxidant status and oxidative stress index values of the amikacin+thymoquinone group were significantly lower than those of the amikacin group. Total antioxidant status values of the amikacin+thymoquinone group were significantly higher than those of the amikacin group.
The toxic effect of certain therapeutic drugs on hearing and vestibular functions is called drug-induced ototoxicity [1]. Drug-induced ototoxicity poses a significant health problem since it resists most medical treatments; however, extensive research on this subject has provided much information about its pathophysiological aspects [2]. Although aminoglycoside antibiotics (AGAs) cover a wide spectrum and have numerous application areas, their side-effects restrict their usage. Currently AGA use is limited to severe infections, especially when a synergistic action is needed, with careful monitoring for side effects [3].
AGAs have been used for the treatment of gram negative infections for a long time. The advantages of those drugs can be listed as; their rapid activation, their low rate of bacterial resistance, their synergistic action with the beta lactams, and their low prices [4]. The toxic effect of AGAs is an important subject in medical research, and the incidence of ototoxicity varies between 10% to 80% according to various studies [5]. The ototoxic effects of AGAs in humans are typically sensorineural, nonsyndromic, bilateral, progressive and affect high frequencies [5]. Inner ear damage caused by those ototoxic agents has not been totally controlled yet.
Amikacin is a semisynthetic aminoglycoside produced by the acetylation of kanamycin A. This structural characteristic renders amikacin resistant to bacterial enzymes which inactivate natural aminoglycosides like gentamicin, kanamycin and tobramycin; thus, amikacin has the widest spectrum of activity among the AGAs [6]. Amikacin causes apoptosis by generating free oxygen radicals, and by the same mechanism, causes drug-induced ototoxicity which functionally manifests as hearing loss [7]. Reactive oxygen species were found to be responsible for the damage AGAs produced in the inner ear [8]. Like the other AGAs, amikacin also has cochleotoxic effects which lead to permanent hearing loss. Many studies have been conducted to find a solution to amikacin-induced hearing loss, but currently there is no consensus on any specific agent for clinical application against amikacin ototoxicity.
Thymoquinone (C10H10O2; 2-isopropyl-5-methyl-1,4-benzoquinone) is the bioactive molecule of Nigella Sativa [9]. N. Sativa is an herb native to the Mediterranean coastal lands; in everyday language it is known as bun's herb, black seed, black cumin, or seed of abundance. Thymoquinone has antioxidant, anti-inflammatory, neuroprotective, antiallergic, antiviral, antidiabetic, and anticancerogenic effects [10]. Thymoquinone possesses a potent scavenger activity for free oxygen radicals, especially for superoxide anions and hydroxyl radicals [11]. The antioxidant effects of thymoquinone are brought forth by the inhibition of cyclooxygenases and lipoxygenases, and by the inhibition of membrane lipid peroxidation [12]. Experimental long-term studies with thymoquinone conducted on rats with did not encounter any toxic effects, and thymoquinone is known to have a wide safety range [13].
The objective of this study is to investigate the protective effects of thymoquinone on experimentally induced amikacin ototoxicity.
After the approval of the Animal Research Local Ethics Committee, 32 healthy female Wistar Albino rats (200-240 g) were included in the study. Rats with a positive Preyer reflex were chosen, endoscopic ear examinations were conducted, and any rat with an outer or middle ear pathology was excluded from the study group. Rats were kept in an environment which was illuminated for 12 hours and darkened for 12 hours and had a temperature of 21℃±1℃, with free access to food and water, and with a background noise below 50 dB. The animals were used in accordance with the Guide for the Care and Use of Laboratory Animals [14]. Rats were assigned to 4 groups with 8 rats in each group: group 1 (amikacin, AK), group 2 (amikacin+thymoquinone, AK+TQ), group 3 (thymoquinone, TQ), group 4 (no treatment group, NTG). Thymoquinone was administered to groups 2 and 3 for 14 days by oral gavage, in a dose of 40 mg/kg/day. Amikacin sulphate was administered intramuscularly to groups 1 and 2 for 14 days, in a dose of 600 mg/kg/day. At the beginning of the study, and on days 7 and 15, all rats were anesthetized by intraperitoneal ketamine hydrochloride (40 mg/kg) and xylazine hydrochloride (5 mg/kg) and DPOAE and ABR measurements were conducted after anesthesia. On the 15th day blood samples were obtained by intracardiac puncture, and biochemical parameters were determined.
The GSI Audera (Viasys Healthcare Inc. Conshohocken, PA, USA) device was used for DPOAE measurements in evaluating the animals' peripheral hearing. The smallest size elastic tympanometry probe was used for the rats. Emissions were performed in General Diagnostic mode, and both DPgram and input-output measurements were taken. Otoacoustic emissions were measured using stimuli in different frequencies and intensities. Primary stimulus intensities were adjusted to 65 dB (L1=L2). The two different frequencies (f1 and f2) were set as f2/f1=1.10. DPgram measurements were performed at 3,000, 4,008, 5,004, 6,000, 6,996, 8,004, 9,012, 10,008, 11,004, and 12,000 Hz frequencies. During measurements, DPOAEs with a noise intensity of 3 dB and over at 2f1-f2 frequency were accepted as positive.
ABR measurements were conducted in a silent room with the Viasys Medelec Synergy (Viasys Healthcare Inc.) device, using subdermal needle electrodes (Technomed Europe, Maastricht-Airport, the Netherlands). ER 3A insert earphones were used to provide click stimuli in alternating polarities. Filter was set at 30-1,500 Hz, repetition rate was set at 21/second, and the time window was set at 25 milliseconds. The 1,024 samples were taken for signal averaging. Initially stimuli were presented at 80 dB normal hearing level intensity, and the intensity level was reduced in 20-dB steps until near-threshold values. Then the intensity level was reduced in 10-dB steps until the threshold value was reached. At least 2 tracks were generated for each measurement to test behavior reproducibility, and the threshold was cross checked. The ABR threshold was defined as the lowest intensity level where the III wave was observed.
Total antioxidant status (TAS) measures the combined activity of antioxidants and the total antioxidant level, thus evaluating the whole antioxidant status [15]. Total oxidant status (TOS) is an indicator of the total oxidant levels [16]. Oxidative stress index (OSI) is calculated by the TOS/TAS ratio, and provides a more accurate index for oxidative stress in the body, since this ratio takes into account the sum total of all oxidant and antioxidant activities.
For biochemical analyses, blood samples obtained from the rats were centrifuged for 15 minutes in 3,000 rpm., and the serum was separated and kept in -80℃ until blood specimens from all the rats were thus prepared. TAS and TOS were measured by the Rel Assay Diagnostics kit (Mega Tip San ve Tic Ltd Sti, Gaziantep, Turkey), and the OSI values were calculated from the TAS and TOS results (OSI: TOS/TASX100).
Statistical analysis was carried out using the SPSS ver. 16.0 (SPSS Inc, Chicago, IL, USA). All quantitative variables were estimated using measures of central location (i.e., mean and median) and measures of dispersion (i.e., standard deviation). Data normality was checked using the Kolmogorov-Smirnov tests of normality. Parametric tests were applied because there was normal distribution of the assessed values.
One-way analysis of variance (ANOVA) was used for inter-group comparisons of DPOAE and ABR values (the difference among groups was considered to be P<0.05). Tukey honest significant difference (HSD) post hoc test was used for determining the differences between the groups (statistical significance was set at P<0.008 for post hoc tests).
Repeated ANOVA test was used for within-group evaluations of DPOAE and ABR values values (the difference among groups was considered to be P<0.05). Bonferroni test was used for evaluating within-group statistical significance (statistical significance was set at P<0.008 for post hoc tests).
One-way ANOVA was used in the evaluation of biochemical parameters since there were 4 independent groups (the difference among groups was considered to be P<0.05). Tukey HSD post hoc test was used for determining which groups differed (statistical significance was set at P<0.008).
In group 1 (AK), DPOAE amplitudes on days 7 and 15 were significantly lower than their initial amplitudes (P<0.05) (Fig. 1). In group 2 (AK+TQ), group 3 (TQ), and group 4 (NTG), there were no significant differences between the initial DPOAE amplitudes and those on days 7 and 15 (P>0.05) (Figs. 2, 3, 4). Inter-group comparisons demonstrated no significant differences between the initial DPOAE amplitudes, while on days 7 and 15, there were significant differences between the DPOAE amplitudes of group 1 (AK) and those of all other groups (P<0.05).
There were significant differences between the initial ABR thresholds of group 1 (AK) and the 7th and 15th day thresholds (P<0.05) (Fig. 5). There were also significant differences between the 7th and 15th day ABR thresholds of group 1 (AK) (P<0.05) (Fig. 5). There were no significant differences between the initial and the 7th and 15th day ABR thresholds of group 2 (AK+TQ), group 3 (TQ), and group 4 (NTG) (P>0.05) (Fig. 5). When the ABR thresholds of days 7 and 15 were compared in group 2 (AK+TQ), group 3 (TQ), and group 4 (NTG), no significant differences were found (P>0.05).
The TAS value of group 2 (AK+TQ) was significantly higher than that of group 1 (AK) (P<0.05) (Table 1). TAS value of group 3 (TQ) was significantly higher than that of group 1(AK) (P<0.05) (Table 1). TAS value of group 4 (NTG) was higher than that of group 1 (AK), but the difference was not statistically significant (P>0.05) (Table 1).
AGA-induced ototoxicity is one of the most common causes of preventable sensorineural hearing loss [17]. Ototoxicity initially affects the outer hair cells located at the basal region of the cochlea, and the resulting hearing loss involves higher frequencies [17]. As the damage progresses, other cochlear turns will also be affected, and lower frequencies, which include human speech frequencies, will be lost to hearing [18].
Many variables, like the dose, routes and periods of administration, total treatment duration and individual differences, affect AGA-induced ototoxicity [19]. Previous studies revealed that a dose of 600 mg/kg/day Amikacin could induce ototoxicity [18], and our study was conducted with that same dose, compatible with relevant literature.
Although AGAs are slowly transported from the blood, they penetrate most body fluids rather rapidly [20]. After injection, AGA enters the inner ear fluid quite quickly [21], and while it is cleared from general circulation in several hours, it stays in the inner ear for several months [22]. Upon entering the inner ear fluids, AGAs exert their ototoxic effects here. AGAs operate their ototoxicity producing effects by generating oxygen free radicals, by lysosomal degradation, inhibition of mitochondrial protein synthesis, calcium activated potassium channel blockade, cellular injury and finally, cell death by apoptosis [23]. After AGAs penetrate the perilymph, their inactive forms interact with iron and become oxygen free radicals which are electron donating molecules. Oxygen free radicals interact with phospholipids, membrane proteins and DNA, and cause irreversible cellular damage, which then manifests as function loss [17]. When the organ of Corti is totally damaged, the destruction may also progress to stria vascularis and the inner ear [24]. In the early phases, while higher frequencies are not yet affected, no signs of the destruction can be detected in pure tone audiometry. At this early phase, DPOAE and ABR are considered more appropriate for hearing assessment [25]; for this reason, we have conducted DPOAE and ABR measurements in our study for audiological evaluation. A high-sensitivity DPOAE device can detect hearing changes in animals brought forth by drugs, before the morphologic changes set in, and the method is objective and noninvasive [26].
Amikacin is a widely used AGA, despite its well known side effects [27]. It is preferred especially for children with febrile neutropenia, for prophylactic purposes and in sepsis, meningitis, bacteremia, urinary tract infections, respiratory tract infections, and against microorganisms resistant to other AGAs.
Numerous in vivo and in vitro studies have demonstrated that Amikacin caused hair cell death and aberration of hearing thresholds and/or hearing levels [28293031323334353637]. In our study, group 1 (AK) had significant decreases of DPOAE amplitudes on days 7 and 15 as compared to initial DPOAE amplitudes, and our findings are compatible with relevant literature. Likewise, the 7th and 15th day ABR thresholds of group 1 (AK) were significantly higher than their initial values, which are also compatible with relevant literature.
Studies with numerous agents were conducted in recent years in search of a prevention for amikacin-induced ototoxicity [28293031323334353637] (Table 2). Research on this issue was accelerated especially during the last decade, but currently there is no agent in use (for counteracting AK's ototoxic effects) that has achieved general consensus. The reasons why chemical agents tested so far could not succeed in establishing a widespread application may be listed as: Different efficacy results in different studies, limited investigation periods to secure long-term usage, probable toxic substances in the contents of some traditional products, high cost, and the limited number of prospective randomized double-blind studies.
During recent years thymoquinone came forward as a much investigated agent, and was shown to have antioxidant, anti-inflammatory, neuroprotective, antiallergic, antiviral, antidiabetic, and anticarcinogenic effects [10]. Thymoquinone is indicated as a potent antioxidant with powerful scavenger activity for free oxygen radicals [11]. A study by Sagit et al. [38] demonstrated thymoquinone's preventive effects on Cisplatin ototoxicity. Another recent study showed the protective effects of thymoquinone against renal injury caused by antituberculosis drugs [39]. In our study, in group 2 (AK+TQ) the DPOAE and ABR values on the 7th and 15th days did not differ significantly from their initial values (Fig. 2), while in group 1 (AK) the 7th and 15th day DPOAE amplitudes were significantly decreased and ABR thresholds significantly increased, compared to their initial values (Fig. 1). Those findings indicate thymoquinone's efficacy in preventing AK ototoxicity.
AK, with its excitotoxic effect produced by the degradation of mitochondrial protein synthesis and overactivation of glutamate receptors (N-methyl-D-aspartate), increases the formation of free radicals and induces apoptosis, and thus generates its ototoxic effect [40]. Most of the agents investigated in medical literature for preventing AK ototoxicity were focused on breaking this cycle, through inhibiting the formation of oxygen free radicals (Table 2). Klemens et al. [41] found a significant correlation with AK-induced hearing loss and the decrease in antioxidant enzymatic action. Our study is a first in exploring the oxidative stress parameters biochemically in a research on the prevention of AK-induced ototoxicity. TOS and OSI were both significantly higher in group 1 (AK) than the other three groups, which indicated that AK did increase oxidative stress (increasing free oxygen radicals, decreasing enzymatic antioxidant activity) (Table 1).
Thymoquinone manifests its potent antioxidant effects through scavenging free oxygen radicals and enhancing enzymatic antioxidant activity [4243]. In our study the TAS values of group 2 (AK+TQ) were significantly higher than those of group 1 (AK) (Table 1). This finding indicates that thymoquinone, with its potent antioxidant effects, counteracted oxidative stress brought about by AK. TAS values in group 3 (TQ) are significantly higher than those of group 1 (AK), which indicates that when TQ is delivered by itself, it increases antioxidant enzymes in the circulation.
A report issued by the World Health Organization noted that drug-induced hearing impairment was a significant health problem, currently being investigated by medical circles, and was an important cause of hearing loss worldwide [44]. Due to their ototoxic effects, AGAs were replaced by various other drugs in the 1980s, but bacterial resistance to those drugs prompted a comeback of AGAs during the last decade [45]. Despite all medical investment for the development of new drugs, only 2 antibiotics have been developed during the last 30 years [46]. In light of the above information, we think that we need to keep using cheap and efficient antibiotics with the least bacterial resistance (like AK), while looking for ways to prevent their undesirable side effects like ototoxicity. The objective of this study targeted to that end, and our findings led us to believe that thymoquinone may be of help in decreasing AK-induced ototoxicity.
The strength of our study lies in its total audiological assessment using DPOAE and ABR tests, in its exploration of the pathophysiological aspects of AK ototoxicity, linking it with the biochemical assessment of oxidative stress, and in the wide safety range of thymoquinone dosage (75-2,600 mg/day) [13], since black cumin seeds have been qualified for the Generally Recognized As Safe inventory in the United States [47]. The main advantages of thymoquinone, which we used in our study, to other chemicals are: according to DPOAE and ABR findings, thymoquinone has completely prevented amikacin ototoxicity.
The weakness of our study is the lack of histopathological evaluation. It has to be kept in mind that ours was an experimental animal study, and further clinical studies, with a prospective double-blinded and placebo controlled study design, are needed for affirmation of our findings.
In conclusion, this study demonstrated that the ototoxic effects of amikacin could be limited by the concurrent use of thymoquinone. Further studies focusing on histopathological findings may reveal this effect more clearly.
References
1. Kasse CA, Hyppolito MA, Cruz OL, de Oliveira JA. Ototoxicity and otoprotection. Rev Bras Med. 2008; 4:105–115.
2. Aquino TJ, Oliveira JA, Rossato M. Ototoxicity and otoprotection in the inner ear of guinea pigs using gentamicin and amikacin: ultrastructural and functional aspects. Braz J Otorhinolaryngol. 2008; Nov-Dec. 74(6):843–852. PMID: 19582340.
3. Avent ML, Rogers BA, Cheng AC, Paterson DL. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J. 2011; 6. 41(6):441–449. PMID: 21309997.

4. Begg EJ, Barclay ML. Aminoglycosides: 50 years on. Br J Clin Pharmacol. 1995; 6. 39(6):597–603. PMID: 7654476.
5. Murillo-Cuesta S, Contreras J, Cediel R, Varela-Nieto I. Comparison of different aminoglycoside antibiotic treatments to refine ototoxicity studies in adult mice. Lab Anim. 2010; 4. 44(2):124–131. PMID: 19858169.

6. Kitasato I, Yokota M, Inouye S, Igarashi M. Comparative ototoxicity of ribostamycin, dactimicin, dibekacin, kanamycin, amikacin, tobramycin, gentamicin, sisomicin and netilmicin in the inner ear of guinea pigs. Chemotherapy. 1990; 36(2):155–168. PMID: 2311443.

7. Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007; 10. 15(5):352–357. PMID: 17823553.

8. Jiang H, Sha SH, Schacht J. NF-kappaB pathway protects cochlear hair cells from aminoglycoside-induced ototoxicity. J Neurosci Res. 2005; 3. 79(5):644–651. PMID: 15672440.
9. Dehkordi FR, Kamkhah AF. Antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008; 8. 22(4):447–452. PMID: 18705755.
10. Ashraf SS, Rao MV, Kaneez FS, Qadri S, Al-Marzouqi AH, Chandranath IS, et al. Nigella sativa extract as a potent antioxidant for petrochemical-induced oxidative stress. J Chromatogr Sci. 2011; 4. 49(4):321–326. PMID: 21439125.

11. Badary OA, Taha RA, Gamal el-Din AM, Abdel-Wahab MH. Thymoquinone is a potent superoxide anion scavenger. Drug Chem Toxicol. 2003; 5. 26(2):87–98. PMID: 12816394.

12. Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol. 2000; 49(1):63–74. PMID: 10997492.
13. Al-Amri AM, Bamosa AO. Phase I safety and clinical activity study of thymoquinone in patients with advanced refractory malignant disease. Shiraz E Med J. 2009; 7. 10(3):107–111.
14. Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council. The guide for the care and use of laboratory animals. 7th ed. Washington DC: National Academies Press;1996.
15. Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004; 4. 37(4):277–285. PMID: 15003729.

16. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; 12. 38(12):1103–1111. PMID: 16214125.

17. Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000; Jan-Feb. 5(1):3–22. PMID: 10686428.

18. Miman MC, Ozturan O, Iraz M, Erdem T, Olmez E. Amikacin ototoxicity enhanced by Ginkgo biloba extract (EGb 761). Hear Res. 2002; 7. 169(1-2):121–129. PMID: 12121745.

19. Lenoir M, Puel JL. Dose-dependent changes in the rat cochlea following aminoglycoside intoxication. II. Histological study. Hear Res. 1987; 26(2):199–209. PMID: 3570998.
20. Xuan W, Dong M, Dong M. Effects of compound injection of Pyrola rotundifolia L and Astragalus membranaceus Bge on experimental guinea pigs' gentamicin ototoxicity. Ann Otol Rhinol Laryngol. 1995; 5. 104(5):374–380. PMID: 7747908.

21. Dulon D, Hiel H, Aurousseau C, Erre JP, Aran JM. Pharmacokinetics of gentamicin in the sensory hair cells of the organ of Corti: rapid uptake and long term persistence. C R Acad Sci III. 1993; 7. 316(7):682–687. PMID: 8019890.
22. Guthrie OW. Aminoglycoside induced ototoxicity. Toxicology. 2008; 7. 249(2-3):91–96. PMID: 18514377.

23. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007; 13(1):119–126. PMID: 17266591.

24. Fausti SA, Henry JA, Schaffer HI, Olson DJ, Frey RH, McDonald WJ. High-frequency audiometric monitoring for early detection of aminoglycoside ototoxicity. J Infect Dis. 1992; 6. 165(6):1026–1032. PMID: 1583319.

25. Stavroulaki P, Vossinakis IC, Dinopoulou D, Doudounakis S, Adamopoulos G, Apostolopoulos N. Otoacoustic emissions for monitoring aminoglycoside-induced ototoxicity in children with cystic fibrosis. Arch Otolaryngol Head Neck Surg. 2002; 2. 128(2):150–155. PMID: 11843723.

26. Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat. Comparison of inner ear tissues and fluids with other organs. J Clin Invest. 1986; 5. 77(5):1492–1500. PMID: 3700652.

27. Sassada MM, Ceccon ME, Navarro JM, Vaz FA. Hearing loss in newborn admitted in intensive care unit. Pediatria (Sao Paul). 2005; 27:163–171.
28. Conlon BJ, Aran JM, Erre JP, Smith DW. Attenuation of aminoglycoside-induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res. 1999; 2. 128(1-2):40–44. PMID: 10082281.
29. Nekrassov V, Sitges M. Vinpocetine protects from aminoglycoside antibiotic-induced hearing loss in guinea pig in vivo. Brain Res. 2000; 6. 868(2):222–229. PMID: 10854574.

30. Oliveira JA, Canedo DM, Rossato M, Andrade MH. Self-protection against aminoglycoside ototoxicity in guinea pigs. Otolaryngol Head Neck Surg. 2004; 9. 131(3):271–279. PMID: 15365547.

31. Erdem T, Ozturan O, Iraz M, Miman MC, Olmez E. Dose-dependent dual effect of melatonin on ototoxicity induced by amikacin in adult rats. Eur Arch Otorhinolaryngol. 2005; 4. 262(4):314–321. PMID: 15170574.

32. Lecain E, Omri B, Behar-Cohen F, Tran Ba, Crisanti P. The role of PKCzeta in amikacin-induced apoptosis in the cochlea: prevention by aspirin. Apoptosis. 2007; 2. 12(2):333–342. PMID: 17191118.
33. Berkiten G, Salturk Z, Topaloglu I, Ugrad H. Protective effect of pentoxifylline on amikacin-induced ototoxicity in rats. Am J Otolaryngol. 2012; Nov-Dec. 33(6):689–692. PMID: 22784588.

34. Amora Lde A, Murashima Ade A, Rossato M, Moreira MB, Hyppolito MA, Fagundes DJ. The effects of hyperbaric oxygen therapy upon ototoxic injuries produced by amikacin in guinea pigs. Braz J Otorhinolaryngol. 2013; May-Jun. 79(3):342–348. PMID: 23743750.
35. Bayindir T, Filiz A, Iraz M, Kaya S, Tan M, Kalcioglu MT. Evaluation of the protective effect of Beta glucan on amikacin ototoxicity using distortion product otoacoustic emission measurements in rats. Clin Exp Otorhinolaryngol. 2013; 3. 6(1):1–6. PMID: 23525870.

36. Pavlidis P, Maurer J, Apostolidou E, Kekes G, Kouvelas D. Memantine's action against aminoglycoside-induced ototoxicity. Eur Arch Otorhinolaryngol. 2014; 6. 271(6):1491–1496. PMID: 23917735.

37. Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000; 6. 97(13):7597–7602. PMID: 10861021.

38. Sagit M, Korkmaz F, Akcadag A, Somdas MA. Protective effect of thymoquinone against cisplatin-induced ototoxicity. Eur Arch Otorhinolaryngol. 2013; 8. 270(8):2231–2237. PMID: 23161274.

39. Jaswal A, Sinha N, Bhadauria M, Shrivastava S, Shukla S. Therapeutic potential of thymoquinone against anti-tuberculosis drugs induced liver damage. Environ Toxicol Pharmacol. 2013; 11. 36(3):779–786. PMID: 23958970.

40. Strupp M, Arbusow V. Acute vestibulopathy. Curr Opin Neurol. 2001; 2. 14(1):11–20. PMID: 11176212.
41. Klemens JJ, Meech RP, Hughes LF, Somani S, Campbell KC. Antioxidant enzyme levels inversely covary with hearing loss after amikacin treatment. J Am Acad Audiol. 2003; 4. 14(3):134–143. PMID: 12859138.

42. Sankaranarayanan C, Pari L. Thymoquinone ameliorates chemical induced oxidative stress and β-cell damage in experimental hyperglycemic rats. Chem Biol Interact. 2011; 4. 190(2-3):148–154. PMID: 21382363.

43. Nagi MN, Al-Shabanah OA, Hafez MM, Sayed-Ahmed MM. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J Biochem Mol Toxicol. 2011; May-Jun. 25(3):135–142. PMID: 20957680.

44. World Health Organization, Programme for the Prevention of Deafness and Hearing Impairment (PDH). Report of an informal consultation on strategies for prevention of hearing impairment from ototoxic drugs. Geneva: World Health Organization;1994.
45. Infectious Diseases Society of America. The 10 × '20 Initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010; 4. 50(8):1081–1083. PMID: 20214473.
46. Norrby SR, Nord CE, Finch R. European Society of Clinical Microbiology and Infectious Diseases. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005; 2. 5(2):115–119. PMID: 15680781.

47. Vanamala J, Kester AC, Heuberger AL, Reddivari L. Mitigation of obesity-promoted diseases by Nigella sativa and thymoquinone. Plant Foods Hum Nutr. 2012; 6. 67(2):111–119. PMID: 22477645.

Fig. 1
Variations in amplitudes of distortion products otoacoustic emissions (DPOAEs) with different time points in group 1 (amikacin). For the comparison within group, the repeated analysis of variance test was applied (P<0.05 was accepted as statistically significant). Preop, preoperation. *To determine the days between which there were differences, the Bonferroni test was administrated as post hoc test (P<0.016 was accepted as statistically significant).
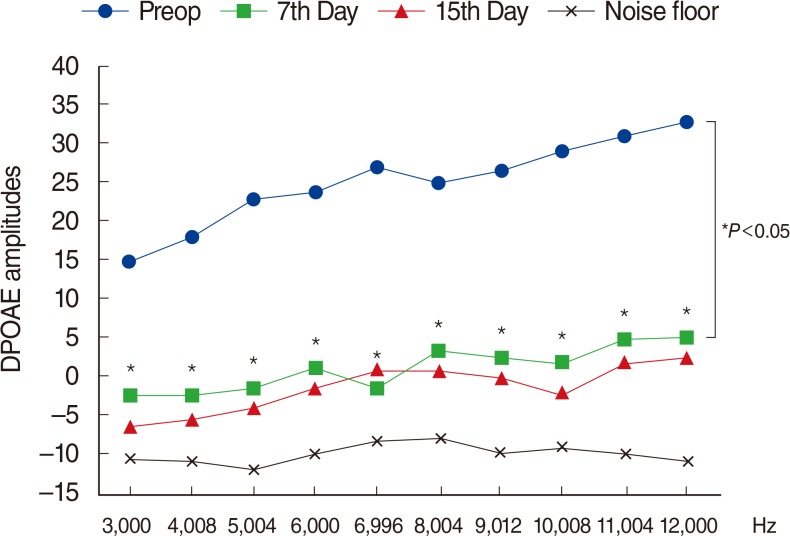
Fig. 2
Variations in amplitudes of distortion product otoacoustic emissions (DPOAEs) with different time points in group 2 (amikacin+thymoquinone). Preop, preoperation. *For the comparison within group, the repeated analysis of variance test was applied (P<0.05 was accepted as statistically significant).
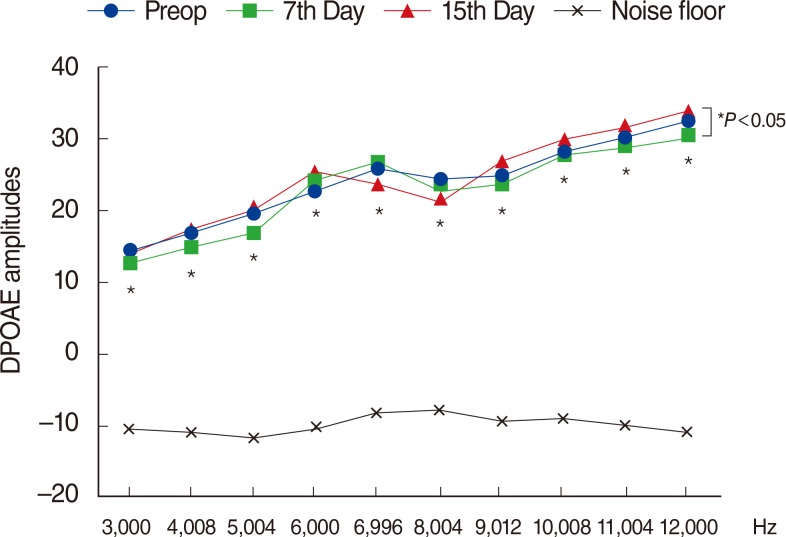
Fig. 3
Variations in amplitudes of distortion product otoacoustic emissions (DPOAEs) in group 3 (thymoquinone) at different time points. Preop, preoperation. *For the comparison within group, the repeated analysis of variance test was applied (P<0.05 was accepted as statistically significant). Preop, preoperation.
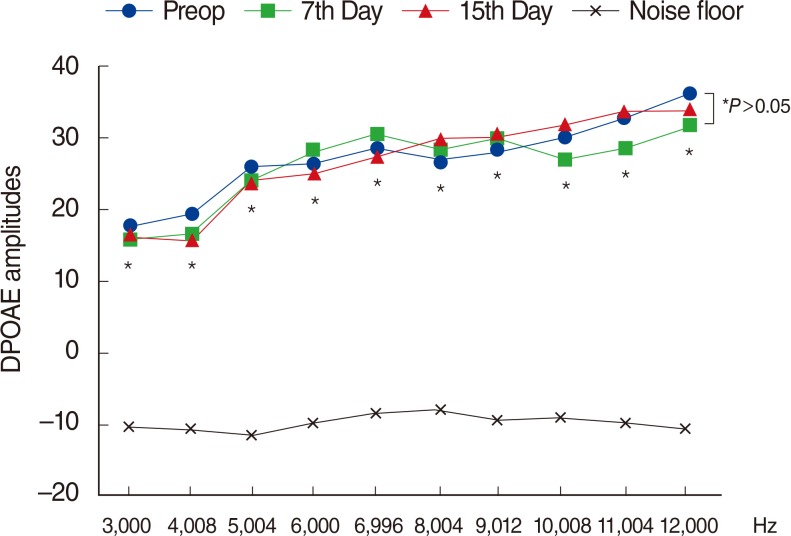
Fig. 4
Variations in amplitudes of distortion product otoacoustic emissions (DPOAEs) with different time points in group 4 (no treatment group). Preop, preoperation. *For the comparison within group, the repeated analysis of variance test was applied (P<0.05 was accepted as statistically significant).
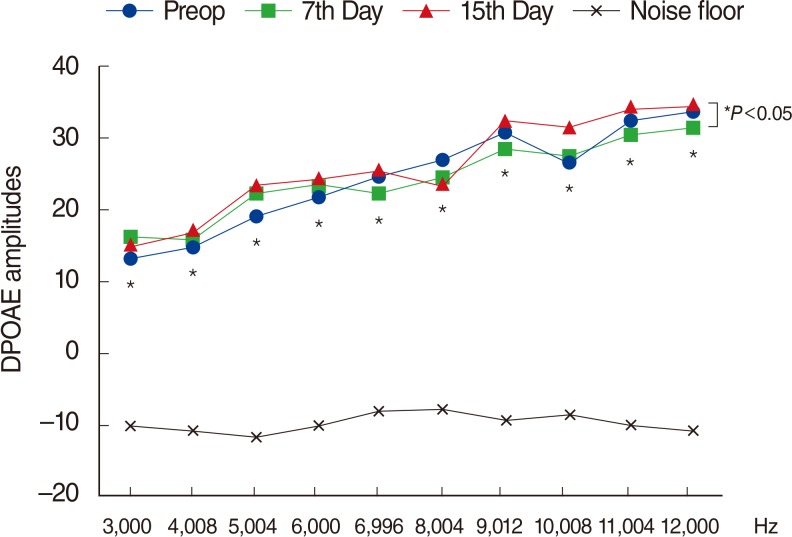
Fig. 5
Variations in amplitudes of auditory brainstem response (ABR) threshold values in all groups at different time points. For the comparison between groups, the one-way analysis of variance test was used (P<0.05 was accepted as statistically significant). nHL, normal hearing level. *Tukey honest significant difference was administrated as post hoc test to identify within-group differences (P<0.008 was accepted as statistically significant).
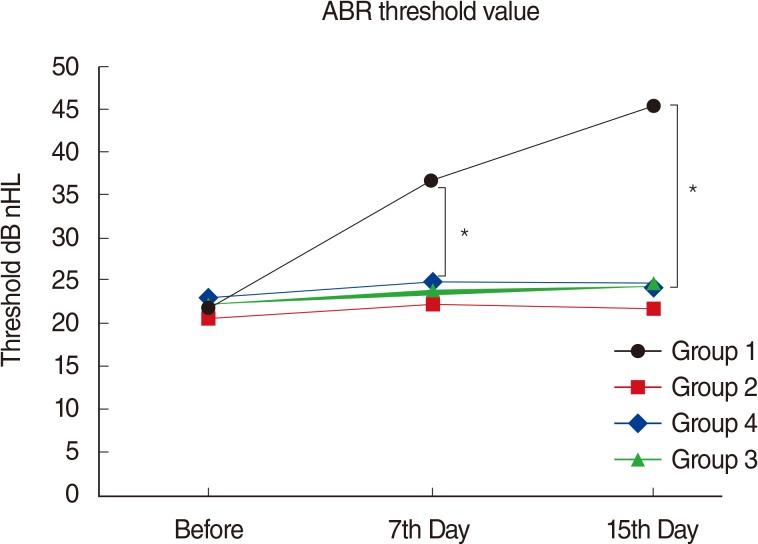
Table 1.
Biochemical parameters
| Variable |
Group (G) |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | G1 vs. G2 | G 1 vs. G3 | G1 vs. G4 | One-way ANOVA* | |
| TOS (μmol H2O2 Eqv/L) | 12.36±0.68 | 8.12±0.99 | 8.66±1.35 | 7.27±1.38 | † | † | † | 0.0001 |
| TAS (μmol Trolox Eqv/L) | 1.05±0.43 | 1.93±0.41 | 2.17±0.55 | 1.54±0.76 | † | † | 0.037 | |
| OSI (TOS/TASX100) | 0.120±0.044 | 0.042±0.007 | 0.039±0.061 | 0.047±0.017 | † | † | † | 0.0001 |
Table 2.
Recent studies focusing on the prevention of amikacin-induced ototoxicity
| Source | Agent/animal | Amikacin (mg/kg/day) | Effect | Evaluation method | Conclusion |
|---|---|---|---|---|---|
| Conlon et al. (1999) [28] | α-lipoic acid/guinea pig | 450 | Free radical scavenger | ABR | Protects the cochlea against hearing loss |
| Duan et al. (2000) [37] | MK801/guinea pig | 300 | NMDA antagonist | ABR | Protects cochlea morphology and physiology |
| Nekrassov et al. (2000) [29] | Vinpocetine/guinea pig | 450 | Blockade of Na+ channels | ABR | Delayed long lasting signs of hearing loss |
| Miman et al. (2002) [18] | Ginko biloba/rat | 600 | Free radical scavenger | DPOAE | Facilitates the development of ototoxicity |
| Oliveira et al. (2004) [30] | Amikacin/guinea pig | 20–400 | Nondamaging-protective dose | SEM | Protects the outer hair cells |
| Erdem et al. (2005) [31] | Melatonin/rat | 600 | Antioxidant/vasodilatory | DPOAE | Low doses protect hair cells, high doses damage hair cells |
| Lecain et al. (2007) [32] | Aspirin/rat | 500 | Prevent PKCexpression | DPOAE+SEM+IHC | Protecting hair cells and spiral ganglion neurons |
| Berkiten et al. (2012) [33] | Pentoxifylline/rat | 200 | Free radical scavenger | DPOAE | Protective effects on hearing functions |
| Amora et al. (2013) [34] | HBO therapy/guinea pig | 600 | Improves oxidative stress | DPOAE+ABR+SEM | Did not change the cochlear hair cell |
| Bayindir et al. (2013) [35] | Beta glucan/rabbit | 600 | Antioxidant | DPOAE | Limit the ototoxic effect of amikacin in the ear |
| Pavlidis et al. (2013) [36] | Memantine/rabbit | 15 | NMDA antagonist | DPOAE | Prevents, at least to some extent, toxic damage |
| Present study | Thymoquinone/rat | 600 | Strong antioxidant | DPOAE+ABR+BCE | Protective effects on hearing and oxidative stress |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download