Abstract
Objectives
In numerous malignancies, angiogenin (ANG) and Maspin are important proangiogenic and antiangiogenic regulators, respectively. The aim of this study was to identify potential relationships between the biological roles of these two proteins in laryngeal squamous cell carcinoma (LSCC).
Methods
Immunohistochemical staining for ANG and Maspin was performed on specimens from 76 consecutive LSCC patients treated with surgery alone, considering the subcellular pattern of Maspin expression. Univariate and multivariate statistical models were used for prognostic purposes.
Results
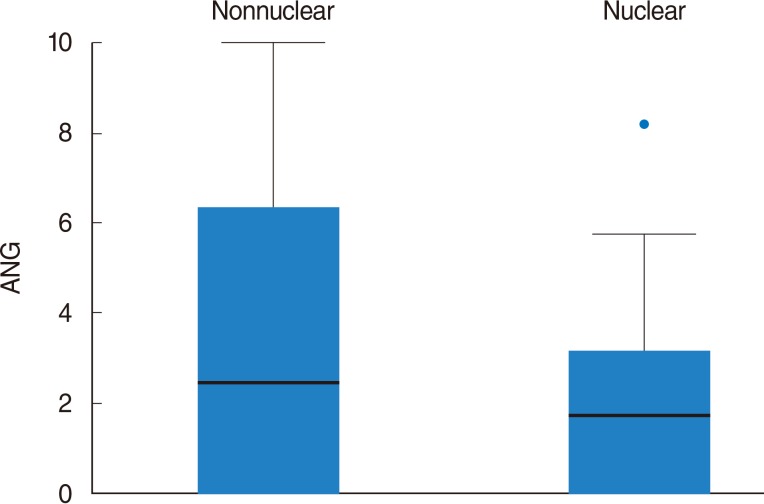
On univariate analysis, a different level of ANG expression was seen for patients stratified by subcellular Maspin expression pattern: the mean ANG expression was higher in cases with a nonnuclear MASPIN expression than in those with a nuclear pattern (P=0.002). Disease-free survival (DFS; in months) differed significantly when patients were stratified by N stage (P=0.01). Patients whose Maspin expression was nonnuclear (i.e., it was cytoplasmic or there was none) had a significantly higher recurrence rate (P<0.001), and shorter DFS (P=0.01) than those with a nuclear Maspin pattern. The mean ANG expression was significantly higher in cases with loco-regional recurrent disease (P=0.007); and patients with an ANG expression ≥5.0% had a significantly shorter DFS than those with an ANG expression <5.0% (P=0.007). On multivariate analysis, ANG expression ≥5.0% was a significant, independent, negative prognostic factor in terms of DFS (P=0.041).
Conclusion
Our results support the hypothesis that a higher ANG expression is associated with a nonnuclear Maspin expression pattern in patients with LSCC. Further studies are needed to clarify the relationship between the ANG and Maspin pathways, and their potential diagnostic and therapeutic role in LSCC.
Angiogenin (ANG) is a member of the ribonuclease superfamily comprising 123 amino acids. Its distinctive structure gives ANG an endothelial binding motif that generates a potent stimulus for neo-angiogenesis in numerous pathophysiological conditions, such as wound healing [1]. ANG has been found upregulated in various types of human cancer, including breast, cervical, colorectal, endometrial, gastric, liver, kidney, ovarian, pancreatic, prostate, and urothelial cancers, and also in astrocytoma, leukemia (acute myeloid leukemia and myelodysplastic syndrome), lymphoma (non-Hodgkin's), melanoma, osteosarcoma and Wilms tumor [2]. ANG is clearly related to the onset, growth, and metastatic spread of tumors, and this was initially attributed to its angiogenic activity [3]. More recent reports have suggested, however, that ANG has an important direct influence on cancer cells, translocating to the nucleus and stimulating rRNA transcription, ribosome biogenesis, proliferation, and tumorigenesis [4].
Another protein that regulates neoplastic angiogenesis is the tumor suppressor Maspin (mammary serine protease inhibitor), a member of the serine protease inhibitor superfamily. Many reports have demonstrated that Maspin has a strong capacity to inhibit angiogenesis. Li et al. [5] provided very detailed molecular information on the antiangiogenic effects of Maspin, showing that it directly induced endothelial cell apoptosis in vitro. They also demonstrated that Maspin overexpression disrupted tumor-induced angiogenesis in vivo (in mice with mammary tumor). Maspin has other antitumor effects too: it inhibits invasion by cancer cells and increases their attachment to the extracellular matrix, and their susceptibility to apoptosis [6]. Maspin may be expressed in the cytoplasm and in the nucleus, and its specific subcellular localization seems to be indicative of its cellular effects, with very important prognostic implications. There is emerging evidence of the pattern of Maspin expression varying not only in different tumor histotypes, but also in the same type of cancer located at different sites [78].
It has been suggested that angiogenesis is essential to tumor growth [9]. ANG and Maspin are important proangiogenic and antiangiogenic regulators, respectively, in several malignancies, but there are no known biological connections between their two pathways. In laryngeal squamous cell carcinoma (LSCC), ANG and Maspin have only been studied separately in terms of how they relate to neo-angiogenesis [1011]. The aim of the present investigation was to study the expression of ANG and the expression and subcellular localization of Maspin and their relations with conventional clinicopathological parameters in a retrospective clinical setting (a series of 76 consecutive LSCCs treated with surgery alone).
The present investigation, approved by the Internal Committee of our Otolaryngology Section, concerned 76 patients with primary LSCC (70 males and 6 females; mean, 63.4±8.3 years; median, 63 years). All patients underwent clinicopathological staging based on endoscopy of the upper aerodigestive tract, neck ultrasonography (with or without fine needle aspiration cytology), contrast-enhanced head and neck computed tomography (CT) and/or magnetic resonance imaging, chest X-ray, liver ultrasonography, microlaryngoscopy with laryngeal biopsy, and esophagoscopy. The patients were treated primarily with either partial laryngectomy (in 61 cases altogether, involving transoral CO2 laser surgery in 21 cases, horizontal supraglottic laryngectomy in 15, and supracricoid laryngectomy in 25) or total laryngectomy (15 cases), always performed by the same surgical team. Staging (Table 1) was based on the 7th edition of the TNM Classification of Malignant Tumors [12]. Unilateral or bilateral curative or elective neck dissections were performed in 58 patients. Postoperative radiotherapy was ruled out for all cases in accordance with current guidelines [13]. No patients presented with distant metastases (M) at diagnosis. The follow-up schedule, adjusted to the patients' characteristics and needs, was: (1) once a month for the 1st year after treatment; (2) every 2 months in the 2nd year; (3) every 3 months in the 3rd year; (4) every 4 months in the 4th year; (5) every 6 months in the 5th year; and (6) every 12 months thereafter. Neck ultrasonography and chest X-rays were performed at least yearly. Contrast-enhanced neck CT, total body positron emission tomography-CT, chest CT, and liver ultrasonography were performed as necessary. All surgical tissues were fixed in 4% paraformaldehyde and embedded in paraffin wax.
Immunohistochemical staining was done using a fully automated system (Bond Max, Leica, Newcastle Upon Tyne, UK). Sections were dewaxed and rehydrated, then incubated in retrieval buffer solution (Leica) for antigen unmasking. The antibodies used were ANG (monoclonal mouse antibody, clone MANG-1, diluted 1:400; AbD Serotec, MorphoSys, Oxford, UK) and Maspin (monoclonal mouse antibody, clone EAW24, diluted 1:100; Leica). Specimens were then washed with phosphate-buffered saline (pH 7.0) and incubated with the Bond Polymer Refine Detection Kit (Leica) according to the manufacturer's protocols. Staining was visualized with 3,3'-diaminobenzidine, and the slides were counterstained with Mayer's hematoxylin. Human placenta and normal breast tissue were used as positive controls for ANG and Maspin staining, respectively. Primary antibodies were replaced with phosphate-buffered solution for negative controls.
The pathologist interpreting the sections (SB) was blinded to the patients' clinical outcomes. For each case, 40 nonoverlapping fields of the less-differentiated areas of SCC, with no evidence of necrosis or hemorrhage, were assessed at ×400 magnification. Considering a minimum of 600 carcinoma cells, the pathologist visually assessed Maspin expression and classified its subcellular distribution pattern as nuclear (almost exclusively nuclear or nuclear and cytoplasmic) or nonnuclear (showing only cytoplasmic reactivity or no reactivity). According to our previous report [14], only the subcellular Maspin distribution pattern is associated with prognosis in LSCC, so in the present investigation the pathologist did not quantify Maspin expression. The same pathologist also measured ANG expression in carcinoma cells from the same areas, estimating the percentage of ANG-stained cells.
The following statistical tests were applied as appropriate: Fisher exact test, Student t-test corrected for unequal variances, and the Mann-Whitney U-test. For prognostic purposes, the main clinicopathological features were binarized as: pT (pT1 vs. pT2-T3), N (pN+ vs. cN0 or pN0), stage (I-II vs. III) and grade (G1-G2 vs. G3). The log-rank test and the Kaplan-Meier product limit estimator were also used to compare disease-free survival (DFS) in months, stratified according to the various parameters analyzed. The receiver operating curve (ROC) approach (failure versus parameter) was used to establish the analytically best-fitting cutoff for binarizing ANG expression according to the highest level of the positive likelihood ratio. The best performance coincides with an area under the ROC (AUC) of 1.0. In the multivariate analysis, Cox proportional hazards regression identified the significant predictors of DFS. A P-value <0.05 was considered significant, while values in the range of 0.10≥P≥0.05 were assumed to indicate a statistical trend. The STATA ver. 8.1 (StataCorp LP, College Station, TX, USA) was used for all analyses.
The mean follow-up, as at June 2013, was 63.6±38.4 months (median, 56 months) (Table 1). Any significant difference in the mean follow-up between patients with and without recurrent disease was ruled out using Student t-test corrected for unequal variances (P=0.93). Fifty-three of the 76 LSCC patients had no recurrent disease after a mean follow-up of 63.3±31.5 months. The other 23 patients developed loco-regional recurrences (local in 19 cases, regional in 3, and both local and regional in 1) after a mean DFS of 16.0±12.0 months (median, 12 months). Five of the 18 cN0 patients who were not treated with an elective dissection developed loco-regional recurrent disease after a mean 24.4±16.3 months (median, 20 months). Fisher exact test was used to identify any differences between the two subgroups of patients with and without loco-regional recurrences, stratified by the main clinicopathological characteristics: it showed a trend towards a significant difference in the patients' distribution by lymph node status (pN+ vs. cN0 or pN0) (P=0.05), but not by pT (P=0.40), stage (P=1.0), or grade (P=0.14). The log-rank test showed a significantly different DFS (in months) when patients were stratified by N stage (P=0.01), but not when they were stratified by pT (P=0.29), stage (P=0.79) or grade (P=0.11).
ANG staining in laryngeal carcinoma cells was seen in 51 of 76 carcinoma samples (67%) (Table 1). The carcinoma cells showed prominent ANG staining of variable intensity in the membrane and cytoplasm (Fig. 1A, C). Statistical analysis failed to identify any significant associations between ANG expression and pT, N (pN+ vs. cN0 or pN0), stage, or grade (Mann-Whitney U-test, P=0.25, P=0.40, P=0.77, and P=0.19, respectively).
The mean ANG expression was significantly higher in the LSCC of patients who developed loco-regional recurrent disease (Mann-Whitney U-test, P=0.007). The analytically best-fitting ANG expression cutoff for prognostic purposes in terms of DFS was 5.0%, calculated using the ROC approach (AUC=0.69; sensitivity 54%, specificity 83%). On statistical analysis, patients with an ANG expression ≥5.0% (38 of 76 patients) had a significantly shorter DFS than patients whose ANG expression was <5.0% (38 patients) (log-rank test, P=0.007) (Fig. 2A).
The pattern of Maspin expression was nuclear in 27 cases (Fig. 1D) and nonnuclear in 49 (Fig. 1B) (Table 1). In the present series, Fisher exact test revealed a trend towards a significant difference in the distribution of the Maspin expression patterns (nuclear vs. nonnuclear) in relation to the variables N (pN+ vs. cN0 or pN0) (P=0.08), and grade (P=0.09), but not pT (P=0.46), or stage (P=0.80).
Patients with a nonnuclear Maspin pattern had a significantly higher recurrence rate (Fisher exact test, P<0.001), and their DFS was significantly shorter (Fig. 2B) (log-rank test, P=0.01).
Student t-test corrected for unequal variances showed different levels of ANG expression in patients stratified by their subcellular Maspin expression pattern: the mean ANG expression was higher in patients with a nonnuclear pattern of Maspin expression than in those with a nuclear pattern (P=0.002) (Fig. 3).
On multivariate analysis, DFS estimates were based on Cox proportional hazards model, assuming no collinearity or interactions between significant variables in the final model. This condition was ascertained by performing a goodness-of-fit test based on Shoenfeld residuals: the model was validated if the P-value for the test indicated no significant deviation from the proportional hazards hypothesis (P=0.20 in our setting). This approach identified N stage, grade, ANG expression, and Maspin pattern as being of potential prognostic value, and their relationship with DFS was estimated. As summarized in Table 2, only ANG expression ≥5.0% (hazard ratio [HR], 2.72; P=0.041) retained its negative prognostic significance in terms of DFS, while a trend towards a negative prognostic significance emerged for N stage (HR, 2.93; P=0.09), and for the nonnuclear Maspin expression pattern (HR, 2.97; P=0.09).
The Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review (1975-2010) on the United States 2010 prevalence counts for invasive cancers reported that laryngeal carcinoma was the 18th most common human malignancy, with 98,063 cases. It was the 12th most common form of cancer in males and came in 20th place for females [15]. Numerous studies [16] have found the clinical characteristics of the tumor, i.e., T stage and N status, associated with prognosis in LSCC, but some head and neck oncologists do not consider these characteristics ideal for establishing an accurate prognosis in all cases of LSCC. In the present investigation, only nodal status was found significantly related to prognosis in terms of DFS on univariate analysis (P=0.01), and this correlation failed to reach a significant value in a multivariate setting. Despite improvements in the diagnosis and treatment of LSCC over the last 40 years, the 5-year relative survival rate has not changed significantly [17]. Given these rather disappointing results, oncological research has focused on the tumor's biology in an effort to shed more light on the different pathways supporting the carcinogenic process. This has led to the identification of molecular markers that may help in the diagnostic process, in prognosis, and in a therapeutic setting too, as targets for specific treatments.
Immunohistochemical staining was used in our investigation to assess the expression of two molecular markers, ANG and Maspin, in LSCC specimens. Our immunohistochemical analyses were easily accomplished within a few days after surgery, on standard paraffin-embedded tissue samples, and immunohistochemistry is currently far less costly and more straightforward than genome profiling. The main strength of our study lies in the homogeneity of the series of patients considered because: (1) they all underwent primary laryngeal surgery alone; (2) their surgical treatment was performed consecutively by the same team; (3) none of the patients required adjuvant treatments according to current guidelines; (4) only surgical specimens (not biopsies) of LSCC were assessed; (5) only squamous cell carcinomas located in a single head and neck structure (the larynx) were considered because the role and subcellular localization of some tumor-related proteins may differ in cancers developing at different head and neck sites (as already seen in the case of Maspin [7]).
The main weaknesses of the present investigation concern its retrospective setting and the limited number of cases considered. In addition, the case series analyzed here (updated from the point of view of the clinical and radiological follow-up) was the object of a previous study by our group on the immunohistochemical expression of Maspin and ANG [18]. The purpose of this earlier investigation was very different, however, and we were not in a position at the time to discuss the biological roles and clinical-pathological relationships between Maspin and ANG in LSCC. There are very few reports on the role of ANG in laryngeal carcinoma [19]. In a series of 108 consecutive patients with LSCC, our research group [10] found that a higher level of ANG expression in carcinoma cells coincided with a higher recurrence rate and a shorter DFS. The present results confirm that a higher mean ANG expression in LSCC cells correlates significantly with a greater likelihood of loco-regional disease recurrence (P=0.007). In the series described here, ANG expression ≥5.0% was also a significant and independent negative prognostic factor in terms of DFS on univariate and multivariate analysis (P=0.007 and P=0.041, respectively).
Using immunohistochemistry, we had previously studied Maspin expression in a large series of consecutive cases of operable LSCC [14], finding that it could be localized in the cytoplasm and in the nucleus, and concluding that patients with a nonnuclear pattern of Maspin expression had a higher recurrence rate and a shorter DFS. Other clinical investigations by our group have focused on the relationships between a nonnuclear Maspin expression pattern in LSCC and more limited antiangiogenic or apoptosis-sensitizing effects [1120]. The present study confirmed our previous findings on the prognostic role of the pattern of Maspin expression in LSCC: a nonnuclear distribution of Maspin was significantly associated with more loco-regional recurrences and a shorter DFS (P<0.001, and P=0.01, respectively). This expression pattern also coincided with a trend towards a negative prognosis, in terms of DFS, on multivariate analysis (P=0.09).
To the best of our knowledge, our research group is the only one to have already investigated the relationship between ANG and Maspin in head and neck cancer, in a series of nasopharyngeal SCC, in which a trend towards an association between the presence of Maspin and a lower ANG expression in carcinoma cells came to light [21]. In the cases of LSCC considered here, patients stratified by subcellular Maspin expression pattern had different levels of ANG expression, i.e., the mean ANG expression in the carcinoma cells was significantly higher for patients with a nonnuclear pattern of Maspin expression than in those with a nuclear pattern (P=0.002). It seems important to emphasize that, judging from our results, a higher ANG expression coincides with the prognostically more negative nonnuclear Maspin pattern. ANG and Maspin are known to have opposite influences on the same two fundamental steps in carcinogenesis: ANG is a proto-oncogene that stimulates tumor neo-angiogenesis [3] and inhibits apoptosis [22], while Maspin is an onco-suppressor with antiangiogenic and proapoptotic effects [6]. Sadagopan et al. [23] recently studied the relationships between ANG and p53 in vitro in normal endothelial cells, transformed human embryonic kidney cells, human osteosarcoma cells, and cancer cell lines (human colon carcinoma, neuroblastoma, hepatocellular carcinoma, and lung adenocarcinoma), and suggested that ANG promotes cell survival by inhibiting the antiapoptotic function of p53. Zou et al. [24] studied the Maspin promoter and identified a consensus p53 site that induced Maspin expression on the binding of p53. Another investigation sought a relationship between p53 levels and Maspin expression in vivo in breast and colon adenocarcinomas, lung cancer, prostatic and ovarian adenocarcinomas, lymphoma, and melanoma, finding that Maspin expression correlated inversely with mutant p53 level in most of these cancers; the authors suggested that Maspin is probably a p53 target gene in vivo [25]. These findings support the hypothesis that ANG inhibits p53, and that p53 regulates the Maspin promoter. In the light of these reports, it would be interesting to study whether ANG over-expression influences the pattern of Maspin expression in LSCC by inhibiting p53 in some way. If so, this connection between the two pathways might provide the rationale for new combinations of targeted therapies for LSCC. Neutralizing monoclonal antibody 26-2F to human ANG was able to prevent PC-3 androgen-independent human prostate cancer from becoming established in treated mice [26]; and an artificial transcription factor specific for the Maspin promoter was able to reactivate Maspin in breast cell lines, and to suppress MDA-MB-231 growth in a xenograft breast cancer model in nude mice [27]. Preclinical trials are needed to test ANG-inhibiting and Maspin-reactivating therapies in models of LSCC to clarify whether there is any real connection between the two pathways and whether a combined therapy could have a synergistic effect.
In conclusion, ANG and Maspin are known to have opposite influences on the same two fundamental steps of carcinogenesis, i.e., neo-angiogenesis and apoptosis, and the relationship between the two has yet to be understood. Our results suggest that higher levels of ANG expression are associated with a nonnuclear pattern of Maspin expression in patients with LSCC. Further studies, involving the protein p53 for instance, are warranted to shed more light on the relationships between the ANG and Maspin pathways, and their diagnostic and therapeutic potential in LSCC.
ACKNOWLEDGMENTS
This study was partly supported by grant No. 60A07-1341/12 (G. Marioni) from the University of Padova, Italy. The authors thank Frances Coburn for correcting the English version of this paper.
References
1. Tello-Montoliu A, Patel JV, Lip GY. Angiogenin: a review of the pathophysiology and potential clinical applications. J Thromb Haemost. 2006; 9. 4(9):1864–1874. PMID: 16961595.

2. Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci U S A. 2006; 9. 103(39):14519–14524. PMID: 16971483.

3. Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005; 1. 24(3):445–456. PMID: 15558023.

4. Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai). 008; 7. 40(7):619–624. PMID: 18604453.
5. Li Z, Shi HY, Zhang M. Targeted expression of maspin in tumor vasculatures induces endothelial cell apoptosis. Oncogene. 2005; 3. 24(12):2008–2019. PMID: 15688005.

6. Bodenstine TM, Seftor RE, Khalkhali-Ellis Z, Seftor EA, Pemberton PA, Hendrix MJ. Maspin: molecular mechanisms and therapeutic implications. Cancer Metastasis Rev. 2012; 12. 31(3-4):529–551. PMID: 22752408.

7. Marioni G, Staffieri A, Blandamura S. Maspin expression in head and neck carcinoma: subcellular localization matters. J Oral Pathol Med. 2010; 3. 39(3):279–280. PMID: 20141575.

8. Gurzu S, Szentirmay Z, Jung I. Molecular classification of colorectal cancer: a dream that can become a reality. Rom J Morphol Embryol. 2013; 54(2):241–245. PMID: 23771065.
9. Marioni G, Giacomelli L, D'Alessandro E, Staffieri C, Guzzardo V, Staffieri A, et al. Laryngeal carcinoma recurrence rate and disease-free interval are related to CD105 expression but not to vascular endothelial growth factor 2 (Flk-1/Kdr) expression. Anticancer Res. 2008; Jan-Feb. 28(1B):551–557. PMID: 18383901.
10. Marioni G, Marino F, Blandamura S, D'Alessandro E, Giacomelli L, Guzzardo V, et al. Neoangiogenesis in laryngeal carcinoma: angiogenin and CD105 expression is related to carcinoma recurrence rate and disease-free survival. Histopathology. 2010; 10. 57(4):535–543. PMID: 20955379.

11. Marioni G, D'Alessandro E, Giacomelli L, De Filippis C, Calgaro N, Sari M, et al. Maspin nuclear localization is related to reduced density of tumour-associated micro-vessels in laryngeal carcinoma. Anticancer Res. 2006; Nov-Dec. 26(6C):4927–4932. PMID: 17214364.
12. Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell;2009.
13. Marioni G, Marchese-Ragona R, Cartei G, Marchese F, Staffieri A. Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev. 2006; 11. 32(7):504–515. PMID: 16920269.

14. Marioni G, Staffieri A, Bertolin A, Giacomelli L, D'Alessandro E, Ottaviano G, et al. Laryngeal carcinoma lymph node metastasis and disease-free survival correlate with MASPIN nuclear expression but not with EGFR expression: a series of 108 cases. Eur Arch Otorhinolaryngol. 2010; 7. 267(7):1103–1110. PMID: 20052590.

15. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, editors. SEER cancer statistics review, 1975-2010 [Internet]. Bethesda (MD): National Cancer Institute;2013. cited 2013 Jun 30. Available from: http://seer.cancer.gov/csr/1975_2010/.
16. Vlachtsis K, Nikolaou A, Markou K, Fountzilas G, Daniilidis I. Clinical and molecular prognostic factors in operable laryngeal cancer. Eur Arch Otorhinolaryngol. 2005; 11. 262(11):890–898. PMID: 15739081.

17. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; Jan-Feb. 62(1):10–29. PMID: 22237781.

18. Marioni G, Blandamura S, Lionello M, Giacomelli L, Lovato A, Favaretto N, et al. Indications for postoperative radiotherapy in laryngeal carcinoma: a panel of tumor tissue markers for predicting locoregional recurrence in surgically treated carcinoma: a pilot study. Head Neck. 2014; 11. 36(11):1534–1540. PMID: 23996283.

19. Marioni G, Blandamura S, Loreggian L, Koussis H, Lionello M, Giacomelli L, et al. Laryngeal carcinoma prognosis after postoperative radiotherapy correlates with CD105 expression, but not with angiogenin or EGFR expression. Eur Arch Otorhinolaryngol. 2011; 12. 268(12):1779–1787. PMID: 21842202.

20. Marioni G, Giacomelli L, D'Alessandro E, Marchese-Ragona R, Staffieri C, Ferraro SM, et al. Nuclear localization of mammary serine protease inhibitor (MASPIN): is its impact on the prognosis in laryngeal carcinoma due to a proapoptotic effect? Am J Otolaryngol. 2008; May-Jun. 29(3):156–162. PMID: 18439947.

21. Marioni G, Koussis H, Scola A, Maruzzo M, Giacomelli L, Karahontziti P, et al. Expression of MASPIN and angiogenin in nasopharyngeal carcinoma: novel preliminary clinico-pathological evidence. Acta Otolaryngol. 2010; 8. 130(8):952–958. PMID: 20105109.

22. Li S, Yu W, Hu GF. Angiogenin inhibits nuclear translocation of apoptosis inducing factor in a Bcl-2-dependent manner. J Cell Physiol. 2012; 4. 227(4):1639–1644. PMID: 21678416.

23. Sadagopan S, Veettil MV, Chakraborty S, Sharma-Walia N, Paudel N, Bottero V, et al. Angiogenin functionally interacts with p53 and regulates p53-mediated apoptosis and cell survival. Oncogene. 2012; 11. 31(46):4835–4847. PMID: 22266868.

24. Zou Z, Gao C, Nagaich AK, Connell T, Saito S, Moul JW, et al. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000; 3. 275(9):6051–6054. PMID: 10692390.

25. Zhang W, Zhang M. Tissue microarray analysis of maspin expression and its reverse correlation with mutant p53 in various tumors. Int J Oncol. 2002; 6. 20(6):1145–1150. PMID: 12011991.

26. Olson KA, Byers HR, Key ME, Fett JW. Inhibition of prostate carcinoma establishment and metastatic growth in mice by an antiangiogenin monoclonal antibody. Int J Cancer. 2002; 4. 98(6):923–929. PMID: 11948474.

27. Beltran A, Parikh S, Liu Y, Cuevas BD, Johnson GL, Futscher BW, et al. Re-activation of a dormant tumor suppressor gene maspin by designed transcription factors. Oncogene. 2007; 4. 26(19):2791–2798. PMID: 17057734.

Fig. 1
(A, B) A patient whose laryngeal squamous cell carcinoma recurred, with a high angiogenin (ANG) expression (A), and a cytoplasmic pattern of Maspin expression (B), in carcinoma cells; (C, D) a patient who had no evidence of disease during the follow-up, with a low ANG expression (C), and a nuclear pattern of Maspin expression (D). (A-D) Immunohistochemical staining.

Fig. 2
(A) Disease-free survival estimates by angiogenin (ANG) expression (%) in laryngeal carcinoma cells (ANG<5.0% or ≥5.0%); (B) disease-free survival estimates by pattern of Maspin expression in laryngeal carcinoma cells (nuclear or nonnuclear); time calculated in months.

Fig. 3
Square root transformed box plot showing the different angiogenin (ANG) expression in patients stratified by subcellular Maspin expression pattern: mean ANG expression was higher in patients with a nonnuclear Maspin expression pattern (nonnuclear) than in those with a nuclear pattern (nuclear).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download