Abstract
Objectives
The aim of this study was to evaluate the prognostic value of volume-based metabolic parameters measured by 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in patients with nasopharyngeal carcinoma (NPC).
Methods
Forty-four NPC patients who underwent 18F-FDG PET/CT for initial staging work-up before concurrent chemoradiotherapy (CCRT) were retrospectively evaluated. Maximum standardized uptake value (SUV), mean SUV, metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of the primary tumors were measured. The prognostic significance and predictive performance of these parameters were assessed by Cox proportional hazards regression analysis and time-dependent receiver operating characteristics (ROC) curve analysis.
Results
Multivariate analysis showed that American Joint Committee on Cancer stage 7th edition (hazard ratio [HR], 1.525; 95% confidence interval [CI], 1.062 to 2.188; P=0.022), and TLG (HR, 7.799; 95% CI, 2.622 to 23.198; P≤0.001) were independent predictive factors associated with decreased disease-free survival (DFS). Time-dependent ROC curve analysis indicated that TLG was a better predictor of DFS than MTV (P=0.008).
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy with unique features that make it epidemiologically, pathologically, and clinically distinct from other head and neck cancers [1]. As this tumor type is highly sensitive to radiation and chemotherapy, concurrent chemoradiotherapy (CCRT) is therefore accepted as the mainstay treatment for locally advanced NPC [2,3]. However, substantial rate of locoregional recurrence and distant metastases have been documented [4]. It is well known that locoregional recurrence and distant metastases are important prognostic factors for overall survival of NPC patients [5,6]. Therefore, identification of high-risk subpopulations of patients with locally advanced disease who might benefit from treatment intensification is of great clinical interest.
Prediction of prognosis in patients with NPC is based on clinical and pathologic features such as TNM stage, tumor histology, radiation dose, presence of cranial nerve involvement [7], and parapharyngeal extension [8]. Among these, TNM stage is the most important and widely used prognostic factor, similar to most other solid tumors. However, recent studies reported that conventional pretreatment primary tumor evaluations may not be sufficient to predict the prognosis of this disease [9,10].
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) has emerged as an important noninvasive imaging modality for staging, response monitoring, and recurrence detection in various types of malignancies [11]. Several studies have reported that the standardized uptake value (SUV) of 18F-FDG PET, which is a semiquantitative metabolic parameter representing glycolytic activity, is associated with prognosis [12,13]. However, it is unclear whether PET parameters can serve as prognostic factors for overall survival or disease-free survival (DFS) in patients with NPC [14,15].
The development of software that automatically calculates volume of interest (VOI) and volume-based metabolic parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) has allowed the computation and evaluation of practical, quantitative PET indices that can serve as prognostic factors [16,17,18]. MTV, which is a volumetric measurement of tumor cells with high glycolytic activity, and TLG, which is the sum SUV within the tumor, have proven useful for predicting prognosis in patients with various types of malignancies, including head and neck cancers [19,20,21,22]. However, the value of volumetric 18F-FDG PET parameters as prognostic factors has not been fully evaluated. In addition, it is unclear which parameter is the best predictor of outcome. Thus, more supportive data are needed to establish the value of these volumetric parameters in predicting prognosis in patients with NPC. To the best of our knowledge, no previous report has investigated and compared the prognostic value of 18F-FDG PET volumetric parameters in NPC patients treated with CCRT. Our aim in this study was therefore to evaluate whether the parameters measured by 18F-FDG PET for initial staging of patients with NPC are prognostic indicators of outcomes in these patients.
Patients with a diagnosis of NPC who underwent 18F-FDG PET/CT for initial staging between October 2004 and February 2009 were identified from the cancer registry of Samsung Medical Center. Eighty consecutive patients were identified and the medical records of these patients were reviewed. Patients who had a pathologically proven carcinoma and who were scheduled to receive CCRT with curative intent were enrolled. Exclusion criteria included a recurrent NPC, secondary primary malignancy, and distant metastasis at the time of initial staging. A total of 44 patients were eligible for inclusion in this study.
Computed tomography (CT) scans of the neck and 18F-FDG PET/CT were performed for initial staging. Abdominal or chest CT scans, neck ultrasonography, and neck magnetic resonance imaging (MRI) were performed when clinically indicated. Based on all information provided by the imaging studies, tumors were staged clinically according to the American Joint Committee on Cancer (AJCC) TNM staging system. The protocol of this retrospective study was reviewed and approved by the Ethics Committee of Samsung Medical Center.
All patients received CCRT with 5-fluorouracil (5-FU)/cisplatin-based regimen for advanced nasopharyngeal cancer based on the intergroup study 0099 guidelines. Patients received cisplatin (100 mg/m2 IV over 2 hours) on day 1 concomitantly with radiotherapy during weeks 1, 4, and 7. Radiotherapy was given using standard fractionation at 1.8 to 2.2 Gy/day and the total dose to the nasopharynx ranged from 66 to 72 Gy. Two weeks after finishing radiotherapy, patients received three cycles of adjuvant chemotherapy consisting of cisplatin (75 mg/m2 IV over 1 hour) on day 1 plus 5-FU (1,000 mg/m2 in a 24-hour continuous infusion) on days 1-4 every three weeks. For emesis prophylaxis, 5-hydroxytryptamine-3 antagonists, substance P antagonists, and dexamethasone were given before and after chemotherapy.
Clinical follow-up examinations including clinical exams, neck CT scans, and/or PET/CT scans were performed every 1-3 months for the first two years, every 5-6 months for the next three years, and annually thereafter. If clinically indicated, other diagnostic work-ups were performed. Recurrence or distant metastasis was diagnosed based on either a positive biopsy or clinical or radiographic evidence of progression.
Patients were instructed to fast for at least six hours before the PET/CT scan. Blood glucose levels were measured before the injection of 18F-FDG and were lower than 200 mg/dL in all patients. PET/CT imaging was performed using one of two dedicated PET/CT scanners (Discovery LS or Discovery STe, GE Healthcare, Milwaukee, WI, USA) without intravenous or oral contrast material. Imaging was performed in 29 of the 44 patients using the Discovery LS PET/CT scanner and in the remaining 15 patients using the Discovery STe PET/CT scanner.
When using the Discovery LS scanner, whole-body CT was performed using a continuous spiral technique with an 8-slice helical CT (140 kVp; 40-120 mA adjusted to the patients' body weight; section width of 5 mm) 45 minutes after the injection of 18F-FDG (5.5 MBq/kg). After the CT scan, an emission scan was obtained from the thigh to head for 4 minutes per frame in 2-dimensional mode. Attenuation-corrected PET images (voxel size, 4.3 mm×4.3 mm×3.9 mm) were reconstructed from the CT data using an ordered-subset expectation maximization algorithm (28 subsets, 2 iterations). When using the Discovery STe scanner, whole-body CT was performed using a continuous spiral technique with 16-slice helical CT (140 kVp; 30-170 mA in AutomA mode; section width of 3.75 mm) 60 minutes after the injection of 18F-FDG (5.5 MBq/kg). After the CT scan, an emission scan was obtained from the thigh to the head for 2.5 minutes per frame in 3-dimensional mode. Attenuation-corrected PET images (voxel size, 3.9 mm×3.9 mm×3.3 mm) were reconstructed from the CT data using a 3-dimensional ordered-subset expectation maximization algorithm (20 subsets, 2 iterations).
Two experienced nuclear medicine physicians reviewed all 18F-FDG PET/CT images for initial staging on a dedicated workstation (GE Advantage Workstation 4.4). Metabolic and volumetric parameters were measured using Volume Viewer software (GE Healthcare, Milwaukee, WI, USA), which provides an automatically delineated VOI using an isocontour threshold method based on the SUV (Fig. 1). MTV was defined as the total tumor volume segmented by the threshold SUV [17]. Mediastinal blood pool (MBP) activity [23] was used as a threshold for determining the VOI boundary. To determine the threshold using MBP, a VOI consisting of 5×5×1 voxels was manually drawn at the aortic arch. Mean standardized uptake value (SUVmean) plus two standard deviations of the VOI in the aortic arch was adopted as the threshold SUV for the primary tumor. Using the threshold SUV, VOIs of the primary tumor were automatically generated. Experienced nuclear medicine physicians manually adjusted VOIs of the primary tumor to exclude adjacent lymph nodes. The software calculated the maximum standardized uptake value (SUVmax), SUVmean, and MTV of the entire primary tumor. TLG was obtained by multiplying the SUVmean by the number of voxels.
Statistical analyses were performed using PASW ver. 18.0 (SPSS Inc., Chicago, IL, USA) and open source statistical software R (http://www.R-project.org). The prognostic significance of continuous PET parameters including SUVmean, SUVmax, MTV, and TLG of the primary tumor and other clinical variables related to DFS was assessed by univariate and multivariate analyses using backward stepwise Cox proportional hazards regression models. Maximal chi-square method was used to select optimal cutoff value of PET parameters. The 'maxstat' package in the R (http://www.R-project.org) was used for the analyses. DFS was defined as the time from the date of CCRT initiation to the date of recurrence or last clinical follow-up. An event was defined as local recurrence or distant metastasis.
Receiver operating characteristics (ROC) curves are frequently used to evaluate the discriminatory power of a continuous variable for a binary disease outcome. However, many disease outcomes are time-dependent. Therefore, time-dependent ROC curves to assess the predictive power of diagnostic markers for time-dependent disease outcomes have been introduced [24]. To further evaluate and compare the predictive performance of the volumetric PET parameters, MTV and TLG, we adapted the time-dependent ROC curve for censored data and used the area under the ROC curve (AUC) as the criterion. The 'survivalROC' and 'survcomp' packages for performance assessment and comparison with time-dependent ROC curve estimation, written using the open-source statistical software R (http://www.R-project.org), was used for the analysis. All tests were two-sided, and P-values less than 0.05 were considered statistically significant.
Patients' demographic and clinical characteristics are summarized in Table 1. During the follow-up period, two patients died because of cancer-related causes (4.5%), while the remaining 42 patients survived (95.5%). Six patients (13.6%) presented with local recurrence and nine patients (20.5%) presented with distant metastasis during the follow-up. Among the patients with distant metastasis, five had lung metastasis, one had liver metastasis, and the others had multiple organ metastases.
The primary lesions of all patients were clearly visible on the initial 18F-FDG PET images. The average SUVmean, SUVmax, MTV, and TLG of the primary lesions of all patients were 4.8±1.3 (range, 2.6 to 7.6), 12.9±4.7 (range, 3.9 to 24.9), 49.5±43.1 cm3 (range, 3.0 to 239.0 cm3), and 440.6±475.6 (range, 10.5 to 2,610.8), respectively.
On univariate analysis, T stage, AJCC stage 7th editon, MTV, and TLG that were analyzed as continuous variables, were significant predictors of DFS (Table 2). SUVmax and SUVmean were not significant predictors of recurrence. To further elucidate the effect of volumetric PET parameters, optimal cutoff values which divide the patients into two groups with different DFS were determined by Maximal chi-square method. The Kaplan-Meier survival curves stratified by TLG and MTV demonstrated significant differences of DFS between subgroups (Fig. 2). Patients with a TLG ≥7,640 or an MTV ≥66 cm3 showed worse DFS.
On multivariate analysis (Table 3), AJCC stage 7th editon and TLG were identified as significant independent prognostic factors associated with decreased DFS. Clinical variables and PET parameters were not significant predictors for overall survival in the present study (data not shown).
Time-dependent ROC curve analysis was used to determine the AUC for each follow-up period (Fig. 3). A larger AUC indicates better predictability of time to an event at a given point in time. Similarly, a larger integrated area under the curve indicates better average predictability of time to event. TLG was a better predictor of DFS than MTV (Fig. 3).
In this study, we demonstrated that TLG measured by 18F-FDG PET/CT for initial staging is an independent prognostic factor for DFS and a better predictor of prognosis than MTV in patients with NPC treated with CCRT only.
18F-FDG PET is a noninvasive functional imaging modality based on tumor glucose metabolism. Because the parameters measured by 18F-FDG PET can provide valuable information regarding the total tumor burden and aggressiveness, these parameters could potentially be used as prognostic factors. Although the prognostic value of these parameters has been evaluated in patients with head and neck cancer, the value of volumetric 18F-FDG PET parameters as prognostic factors has not established [9,13,21,22]. Therefore, considering that tumor type and treatment are strongly associated with prognosis, we investigated the prognostic value of 18F-FDG PET parameters in patients with NPC treated with CCRT only.
SUVmax is the metabolic index most commonly used to assess tumor activity in clinical practice because it is an observer-independent measurement. It has been shown that SUVmax is a valuable tool for predicting treatment response and survival in patients with head and neck cancer and other tumor types [25]. However, SUVmax, a single voxel value susceptible to noise, may not accurately reflect the overall tumor burden [26]. A recent study of NPC patients treated with radiotherapy or CCRT reported that SUVmax of the primary tumor was not a significant independent prognostic factor [9]. This is consistent with the results of previous studies that documented that SUVmax of the primary tumor was not an independent prognostic factor for survival and was a poor predictor of treatment outcome [21].
In contrast to SUVmax, SUVmean indicates the average intensity of 18F-FDG uptake by the entire tumor mass and therefore provides more information about the metabolic activity of the entire tumor than SUVmax. A recent study reported that the SUVmean of the primary tumor before treatment was associated with DFS and may be a useful prognostic parameter [13]. However, SUVmean of the primary tumor was not a useful prognostic parameter in our study. This discrepancy may be due to confounding factors related to treatment [13]. Moreover, this parameter has limited reproducibility because it is highly dependent on how the region of interest is drawn [26]. Therefore, in future studies, attempts should be made to minimize the variability of 18F-FDG scan measurements of SUVmean.
Tumor volume is known to be a significant predictor of treatment response. A previous study suggested that the volume of the primary tumor should be considered as an additional prognostic factor in NPC [27]. In current clinical practice, the volume of the target tumor measured by CT or MRI is used to determine tumor size [28]. However, measurement of the real tumor size or tumor burden based on anatomical imaging only is subject to error if the tumor has an irregular shape, heterogeneous composition, and/or vague boundaries. The more important point is that size or volume as calculated by anatomical imaging is only a rough surrogate marker of underlying tumor burden.
The commercial availability of volumetric analysis tools that automatically calculate VOI using an isocontour threshold method without interobserver variability have led to the routine use of volumetric 18F-FDG PET measurements in clinical practice [29]. Volume-based PET parameters such as MTV and TLG represent the total volume and activity of metabolically active tumor cells, respectively. Theoretically, MTV and TLG are more reliable indices than single pixel values. However, it is not clear whether MTV or TLG is a better predictor of survival in patients with head and neck cancers. Previous studies reported that MTV was an adverse prognostic factor for overall survival, independent of other established prognostic factors [21,30]. In the current study, MTV was not an independent prognostic factor on multivariate survival analysis. This discrepancy between our study and the previous studies may be due to the relatively low number of subjects evaluated in our study. Therefore, we cannot exclude the possibility that MTV is a significant prognostic factor for DFS in patients with NPC.
Another studies demonstrated that TLG is more valuable for predicting long-term survival than MTV and SUV in patients with nasopharyngeal cancer [22]. TLG, which is a combination of SUVmean and MTV, simultaneously indicates the degree of 18F-FDG uptake and the size of the metabolically active tumor. TLG may therefore be the ideal metabolic parameter to reflect total tumor burden. In support of this, TLG was the only independent prognostic factor of DFS on multivariate survival analysis among the 18F-FDG PET parameters that we evaluated. Furthermore, in our study, TLG was a better predictor of DFS than MTV based on time-dependent ROC curve analysis of patients with NPC.
Our study had several limitations. The low number of subjects we evaluated and the retrospective study design limit the extent to which our results can be generalized. Inconsistencies between our results and those of previous studies may be due to the relatively low number of subjects that we evaluated. Another limitation is that possible risk factors, such as Epstein-Barr virus status and DNA level, were not included in the analysis due to limited cases with available data. The short follow-up duration is also a limitation of our study. Although larger volumetric parameter values were significantly associated with locoregional recurrence or distant metastasis, there was no significant correlation between the parameters and overall survival. The reason may be that the follow-up duration in this study is relatively short to observe cancer-related death because patients with NPC have a good survival rate, even in the advanced stage of disease. Long-term prospective validation studies of large populations are required to confirm our findings. Despite the limitations of this study, the results obtained from this study strongly suggest that TLG may be an independent predictor of prognosis in patients with NPC.
In conclusion, we showed that the pretreatment TLG of a primary tumor, which is a volumetric parameter of 18F-FDG PET, is an important independent prognostic factor and is a better predictor of DFS than MTV in patients with NPC. These results suggest that TLG is a potentially valuable tool for risk stratification and treatment decision. In patients with advanced NPC and high TLG, treatment intensification may be justified, and these patients should be closely followed-up because of the high risk of locoregional recurrence or distant metastasis. Additional large-scale prospective studies are needed to validate the prognostic utility of this promising 18F-FDG PET biomarker.
ACKNOWLEDGMENTS
This study was supported by grant from the Samsung Medical Center Clinical Research Development Program grant #CRS-111-17-1 and grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare (A110568), Republic of Korea.
References
1. Al-Sarraf M, Reddy MS. Nasopharyngeal carcinoma. Curr Treat Options Oncol. 2002; 2. 3(1):21–32. PMID: 12057084.
2. Kim YS, Kim BS, Jung SL, Lee YS, Kim MS, Sun DI, et al. Radiation therapy combined with (or without) cisplatin-based chemotherapy for patients with nasopharyngeal cancer: 15-year experience of a single institution in Korea. Cancer Res Treat. 2008; 12. 40(4):155–163. PMID: 19688124.
3. Zhang L, Zhao C, Ghimire B, Hong MH, Liu Q, Zhang Y, et al. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: a meta-analysis of the phase III randomized trials. BMC Cancer. 2010; 10. 10:558. PMID: 20950416.

4. Lu H, Peng L, Yuan X, Hao Y, Lu Z, Chen J, et al. Concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a treatment paradigm also applicable to patients in Southeast Asia. Cancer Treat Rev. 2009; 6. 35(4):345–353. PMID: 19211192.

5. Liu MT, Hsieh CY, Chang TH, Lin JP, Huang CC, Wang AY. Prognostic factors affecting the outcome of nasopharyngeal carcinoma. Jpn J Clin Oncol. 2003; 10. 33(10):501–508. PMID: 14623917.

6. Farias TP, Dias FL, Lima RA, Kligerman J, de Sa GM, Barbosa MM, et al. Prognostic factors and outcome for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2003; 7. 129(7):794–799. PMID: 12874084.

7. Lee AW, Law SC, Foo W, Poon YF, Cheung FK, Chan DK, et al. Retrospective analysis of patients with nasopharyngeal carcinoma treated during 1976-1985: survival after local recurrence. Int J Radiat Oncol Biol Phys. 1993; 8. 26(5):773–782. PMID: 8344845.

8. Sham JS, Choy D. Prognostic value of paranasopharyngeal extension of nasopharyngeal carcinoma on local control and short-term survival. Head Neck. 1991; Jul-Aug. 13(4):298–310. PMID: 1869431.

9. Liu WS, Wu MF, Tseng HC, Liu JT, Weng JH, Li YC, et al. The role of pretreatment FDG-PET in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012; 2. 82(2):561–566. PMID: 21300483.

10. Chan SC, Chang JT, Wang HM, Lin CY, Ng SH, Fan KH, et al. Prediction for distant failure in patients with stage M0 nasopharyngeal carcinoma: the role of standardized uptake value. Oral Oncol. 2009; 1. 45(1):52–58. PMID: 18487079.

11. Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008; 3. 49(3):480–508. PMID: 18287273.

12. Schwartz DL, Rajendran J, Yueh B, Coltrera MD, Leblanc M, Eary J, et al. FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004; 12. 130(12):1361–1367. PMID: 15611393.

13. Higgins KA, Hoang JK, Roach MC, Chino J, Yoo DS, Turkington TG, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int J Radiat Oncol Biol Phys. 2012; 2. 82(2):548–553. PMID: 21277108.

14. Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004; 8. 59(5):1295–1300. PMID: 15275712.

15. Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007; 10. 69(2):328–333. PMID: 17869659.

16. Choi KH, Yoo IeR, Han EJ, Kim YS, Kim GW, Na SJ, et al. Prognostic value of metabolic tumor volume measured by (18)F-FDG PET/CT in locally advanced head and neck squamous cell carcinomas treated by surgery. Nucl Med Mol Imaging. 2011; 3. 45(1):43–51. PMID: 24899977.

17. Hyun SH, Choi JY, Shim YM, Kim K, Lee SJ, Cho YS, et al. Prognostic value of metabolic tumor volume measured by 18F-fluorodeoxyglucose positron emission tomography in patients with esophageal carcinoma. Ann Surg Oncol. 2010; 1. 17(1):115–122. PMID: 19826877.

18. Moon SH, Choi JY, Lee HJ, Son YI, Baek CH, Ahn YC, et al. Prognostic value of 18F-FDG PET/CT in patients with squamous cell carcinoma of the tonsil: comparisons of volume-based metabolic parameters. Head Neck. 2013; 1. 35(1):15–22. PMID: 22307893.
19. Kim BS, Kim IJ, Kim SJ, Nam HY, Pak KJ, Kim K, et al. The prognostic value of the metabolic tumor volume in FIGO stage IA to IIB cervical cancer for tumor recurrence: measured by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2011; 3. 45(1):36–42. PMID: 24899976.

20. Lee HY, Hyun SH, Lee KS, Kim BT, Kim J, Shim YM, et al. Volume-based parameter of 18)F-FDG PET/CT in malignant pleural mesothelioma: prediction of therapeutic response and prognostic implications. Ann Surg Oncol. 2010; 10. 17(10):2787–2794. PMID: 20461469.
21. La TH, Filion EJ, Turnbull BB, Chu JN, Lee P, Nguyen K, et al. Metabolic tumor volume predicts for recurrence and death in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2009; 8. 74(5):1335–1341. PMID: 19289263.

22. Chan SC, Chang JT, Lin CY, Ng SH, Wang HM, Liao CT, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun. 2011; 11. 32(11):989–996. PMID: 21862944.
23. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007; 2. 25(5):571–578. PMID: 17242397.

24. Zheng Y, Cai T, Feng Z. Application of the time-dependent ROC curves for prognostic accuracy with multiple biomarkers. Biometrics. 2006; 3. 62(1):279–287. PMID: 16542256.

25. Sasaki R, Komaki R, Macapinlac H, Erasmus J, Allen P, Forster K, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005; 2. 23(6):1136–1143. PMID: 15718309.

26. Soret M, Bacharach SL, Buvat I. Partial-volume effect in PET tumor imaging. J Nucl Med. 2007; 6. 48(6):932–945. PMID: 17504879.

27. Chu ST, Wu PH, Chou P, Lee CC. Primary tumor volume of nasopharyngeal carcinoma: prognostic significance for recurrence and survival rate. Eur Arch Otorhinolaryngol. 2008; 7. 265(Suppl 1):S115–S120. PMID: 18236065.

28. Takes RP, Rinaldo A, Silver CE, Piccirillo JF, Haigentz M Jr, Suarez C, et al. Future of the TNM classification and staging system in head and neck cancer. Head Neck. 2010; 12. 32(12):1693–1711. PMID: 20191627.

29. Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013; Jan-Feb. 14(1):1–12. PMID: 23323025.

30. Lee SJ, Choi JY, Lee HJ, Baek CH, Son YI, Hyun SH, et al. Prognostic value of volume-based (18)F-fluorodeoxyglucose PET/CT parameters in patients with clinically node-negative oral tongue squamous cell carcinoma. Korean J Radiol. 2012; Nov-Dec. 13(6):752–759. PMID: 23118574.
Fig. 1
Initial 18F-fluorodeoxyglucose (FDG) positron emission tomography images of a nasopharyngeal carcinoma in a 73-year-old female patient. (A) The increased FDG uptake by the primary tumor in the nasopharynx is clearly visible in the maximum intensity projection image. A volume of interest (VOI) was automatically placed over the primary tumor using an isocontour threshold method. Segmented VOIs are shown on the transverse (B), sagittal (C), and coronal (D) images.
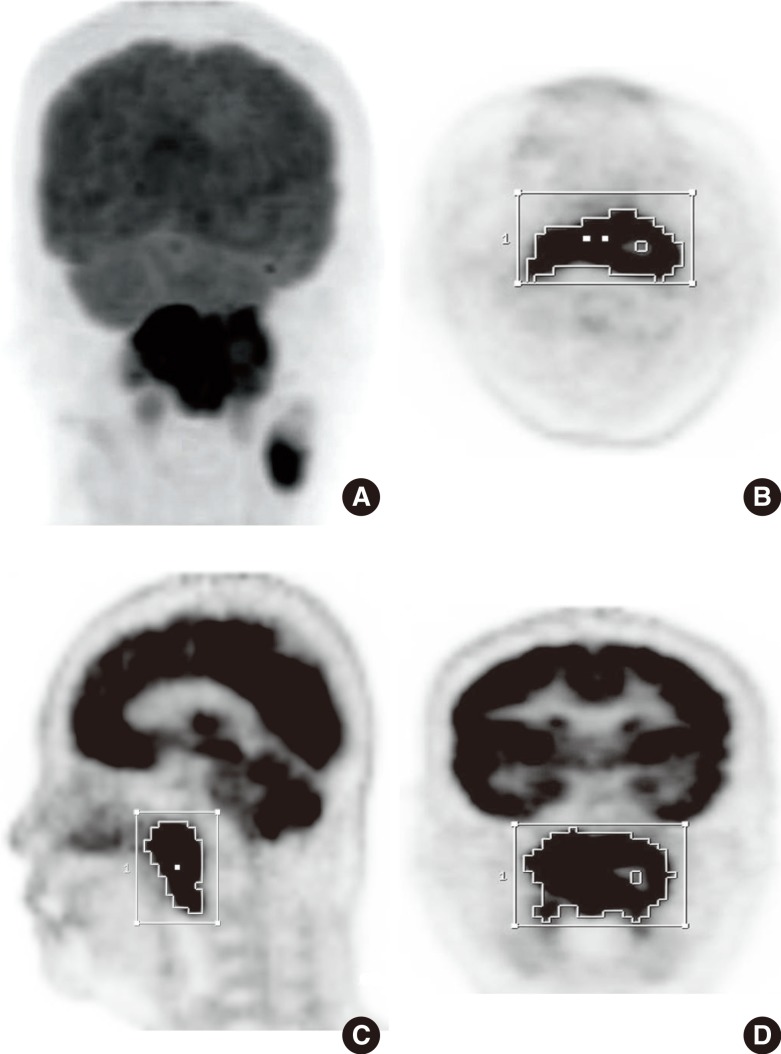
Fig. 2
Disease-free survival stratified by total lesion glycolysis (TLG) (A) and metabolic tumor volume (MTV) (B) in patients with nasopharyngeal carcinoma treated with concurrent chemoradiotherapy.
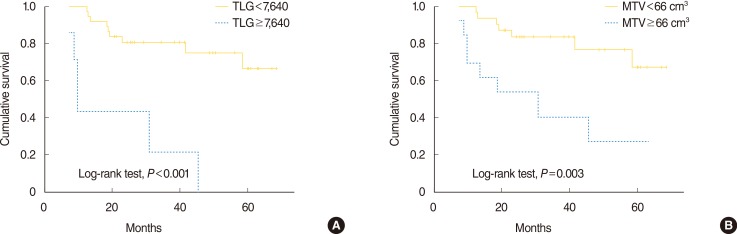
Fig. 3
Time-dependent receiver operating characteristics curve analysis for disease-free survival prediction according to MTV (continuous variable) and TLG (continuous variable). Integrated area under the curve (IAUC) of TLG and MTV were 0.768 and 0.749, respectively. The IAUC of TLG was significantly larger than that of MTV (P<0.008). MTV, metabolic tumor volume; TLG, total lesion glycolysis.
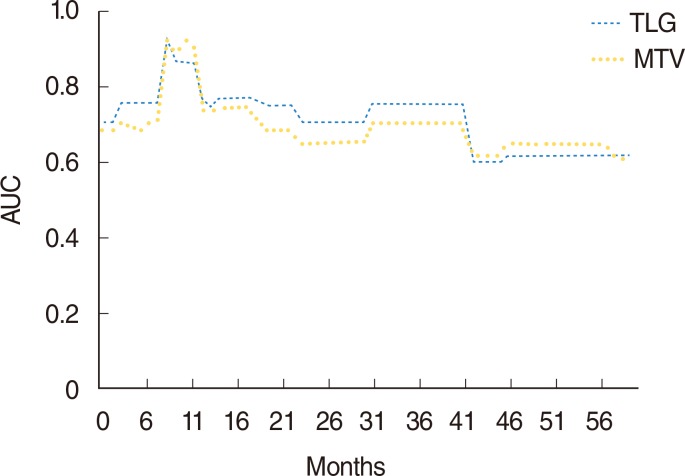
Table 1.
Patient demographics and clinical characteristics (n=44)
Table 2.
Univariate analysis for DFS using a Cox proportional hazards model
| Variable | HR | 95% CI | P-value |
|---|---|---|---|
| Age (10-year interval) | 0.835 | 0.572–1.218 | 0.348 |
| Sex (male vs. female) | 1.257 | 0.354–4.469 | 0.723 |
| T classification (T1–T4) | 1.696 | 1.269–2.876 | 0.049* |
| N classification (N0/N1–N3) | 1.013 | 0.499–2.056 | 0.972 |
| AJCC stage 7th edition (0-I/II/III/IV) | 1.520 | 1.062–2.175 | 0.022* |
| Histopathology (D vs. UD) | 0.308 | 0.081–1.170 | 0.084 |
| SUVmax (1 unit increase) | 1.026 | 0.920–1.114 | 0.650 |
| SUVmax ≥7.8 | 26.754 | 0.071–1.0×104 | 0.278 |
| SUVmean (1 unit increase) | 1.351 | 0.888–2.055 | 0.160 |
| SUVmean ≥4.0 | 5.162 | 0.673–39.574 | 0.114 |
| MTV (1-cm3 increase) | 1.008 | 1.001–1.016 | 0.033* |
| MTV≥66 cm3 | 4.141 | 1.487–11.535 | 0.007* |
| TLG (10 unit increase) | 1.001 | 1.000–1.002 | 0.007* |
| TLG≥7,640 | 7.624 | 2.610–22.272 | <0.001* |
DFS, disease-free survival; HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; D, nonkeratinizing differentiated; UD, nonkeratinizing undifferentiated; SUVmax, maximum standardized uptake value; SUVmean, mean standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download