Abstract
Objectives
This study evaluated the risk factors for anastomotic leakage (AL) and survival outcomes in patients with head and neck squamous cell carcinoma (HNSCC).
Methods
Patients with HNSCC who underwent surgery carrying potential AL from 2003 through 2009 were included in this study. Univariate and multivariate analyses were performed and patient survival was calculated by the Kaplan-Meier method.
Results
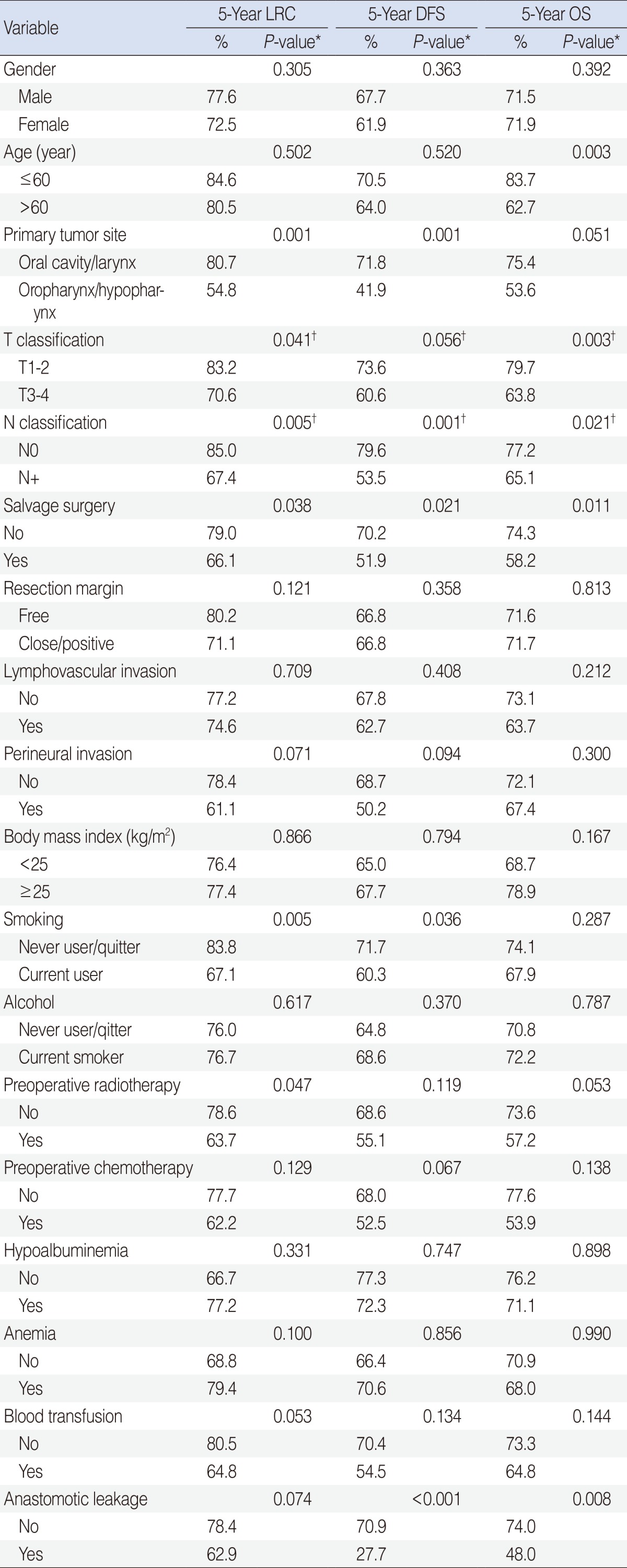
Of 232 eligible patients, 25 (10.8%) developed AL. Univariate analyses revealed that primary tumor site, salvage surgery, perineural invasion, radiotherapy, chemotherapy, and blood transfusion were significantly associated with the occurrence of AL (P<0.05). Independent risk factors for AL were salvage surgery and blood transfusion (P<0.01). On univariate analysis, AL was significantly associated with overall (OS) and disease-free survivals (DFS; P<0.05) but not with decreased locoregional control (LRC) rate (P=0.07). The 5-year DFS rate was significantly different between the non-leakage and leakage groups (70.9% vs. 27.7%, P<0.001). Multivariate analysis showed, however, that AL was not an independent variable of LRC, DFS, or OS (P>0.1).
Anastomotic leakage (AL) from the upper aerodigestive tract is a serious complication after surgery for head and neck cancer, with a reported incidence of 5% to 65% [1-9]. Although surgical techniques and perioperative management have significantly improved in recent decades, AL remains a major problem, leading to significant morbidity, such as salivary leakage, prolonged hospitalization, swallowing problems, delayed initiation of adjuvant therapy, potential carotid artery rupture, and sepsis [10]. The causes of AL are multifactorial, and high-risk patients can be characterized by flap reconstruction, poor nutritional status, infection, postoperative hemoglobin levels, prolonged operative time, transfusion, and radiotherapy [7].
Many causative factors associated with AL after surgery for head and neck cancer have been described, but remain under debate [4-11]. Postoperative wound infections may affect locoregional control (LRC) and/or survival outcomes in patients undergoing major surgery for head and neck cancer [12]. Although many reports evaluate the incidence and risk factors for AL in patients with head and neck squamous cell carcinoma (HNSCC), it is necessary to redefine them in light of the predictions in prognosis of AL and advances in therapeutic modalities. We believe that updated reports on AL occurrence in HNSCC patients may lead to improved prediction and detection of AL and appropriate management of HNSCC patients. Therefore, we sought to examine the incidence, risk factors, and survival outcomesassociated with AL occurrence in HNSCC patients.
This study included consecutive patients with HNSCC who underwent surgery from January 2003 through December 2009 at the Asan Medical Center. This study was reviewed and approved by the Institutional Review Board of the Asan Medical Center. Included patients underwent definitive surgery consisting of gross complete tumor resection with/without reconstruction carrying potential AL, such as flap reconstruction, open partial or total laryngectomy, and pharyngectomy for HNSCC arising in the oral cavity, oropharynx, larynx, and/or hypopharynx. Exclusion criteria were patients who underwent transoral surgery and primary closure after HNSCC surgery, patients less than 18 years of age, patients with preoperative recurrences or second cancers, and patients with distant metastasis or palliative treatments. Salvage surgery was done in case of persistent disease after concurrent chemoradiotherapy. We excluded cases which diagnosed as complete remission state before recurrence of disease. All patients underwent routine neck dissection as follows: selective neck dissection for a clinically negative neck depending on the primary tumor site and modified or radical neck dissection for a clinically positive neck. AL in this study was suspected as wound erythema in combination with neck and facial edema with soft tissue swelling, fever, and discharge of pus, or gas from the drain. All ALs were confirmed by one or more of the following methods in addition to the clinical signs: contrast-enhanced head and neck computed tomography (CT) scan, esophagography with barium swallowing, and/or reoperation for suspicious fistulae [7,13].
The body mass index (BMI) each patient was calculated as weight (kg)/height (m2). Data obtained from medical records included patient age and gender, smoking and alcohol history, tumor and nodal staging, pathology, and treatments. Preoperative radiotherapy, preoperative chemotherapy were performed for curative purpose. Laboratory findings including serum albumin and hemoglobin concentrations were also obtained. Hypoalbuminemia was defined as a serum albumin concentration below 3.3 g/dL and anemia as a hemoglobin concentration below 13 g/dL in men and below 12 g/dL in women. Involved resection margins were defined as positive or close (<5 mm). The stage was based on the tumor-node-metastasis (TNM) classification according to the American Joint Committee on Cancer (AJCC) staging classification (6th ed.) [14]. Surveillance for recurrence was regularly performed following surgery by physical examination, head and neck CT/magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) or combined 18F-FDG PET/CT.
Statistical analyses were performed using the SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). Results are presented as means, medians with ranges, or percentages. Continuous variables were compared by the Mann-Whitney U-test, and categorical variables between two groups were compared using two-sided Fisher exact or the χ2 test. Multivariate analysis was performed by binary logistic regression of variables with P<0.05 on univariate analysis. LRC and survival rates were calculated using the Kaplan-Meier method and compared using the log-rank test to identify survival outcomes according to causal factors. The Cox-proportional hazard model was used for multivariate comparisons. Two-sided P-value<0.05 was regarded as statistically significant. Overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up, and disease-free survival (DFS) was measured from the date of surgery to the date of tumor recurrence or last follow-up.
Eligible patients included 199 men and 33 women with a median age of 62 years (range, 20 to 81 years). The clinicopathologic characteristics of all patients are summarized in Table 1. AL occurred in 25 of the 232 patients (10.8%). The time elapsed from surgery to the detection of AL was a median of 8 days (range, 1 to 25 days). AL was more frequently seen in the oropharynx (27.8%) and hypopharynx (25.0%) than in the oral cavity (5.2%) or larynx (8.8%; P=0.007). No significant differences in gender, age, tumor or nodal stage, site of tumor, or type of surgery were found between the non-leakage and leakage groups.
When the factors predictive of AL were analyzed, primary tumor site, salvage surgery and perineural invasion, preoperative radiotherapy, preoperative chemotherapy, and blood transfusion were significantly associated with the occurrence of AL in HNSCC patients (P<0.05) (Table 2). However, there was no significant difference between the non-leakage and leakage groups with respect to age, T or N classification, resection margin status, lymphovascular invasion, BMI, smoking, alcohol, postoperative albuminemia, or postoperative anemia (P>0.1). Multivariate analysis revealed that independent risk factors of AL included salvage surgery (P=0.008; odds ratio [OR], 7.579; 95% confidence interval [CI], 1.688 to 34.022) and blood transfusion (P=0.002; OR, 4.480; 95% CI, 1.723 to 11.650) (Table 3).
We compared survival outcomes according to clinicopathologic variables using univariate and multivariate analyses. On univariate analysis, primary tumor sites, T or N classification, salvage surgery, smoking, preoperative treatment, and radiotherapy were found to be related to the LRC rate (P<0.05). In addition, primary tumor sites, N classification, salvage surgery, smoking, and AL were significantly correlated with low DFS (P<0.05). T or N classification, salvage surgery, and AL were associated with low OS (P<0.05). Independent variables on multivariate analysis of the LRC rate using the Cox-proportional hazard model revealed that primary tumor site, T or N classification, salvage surgery, and preoperative radiotherapy were included. However, T or N classification was an independent variable on multivariate analysis of DFS and OS using the Cox-proportional hazard model (Table 4).
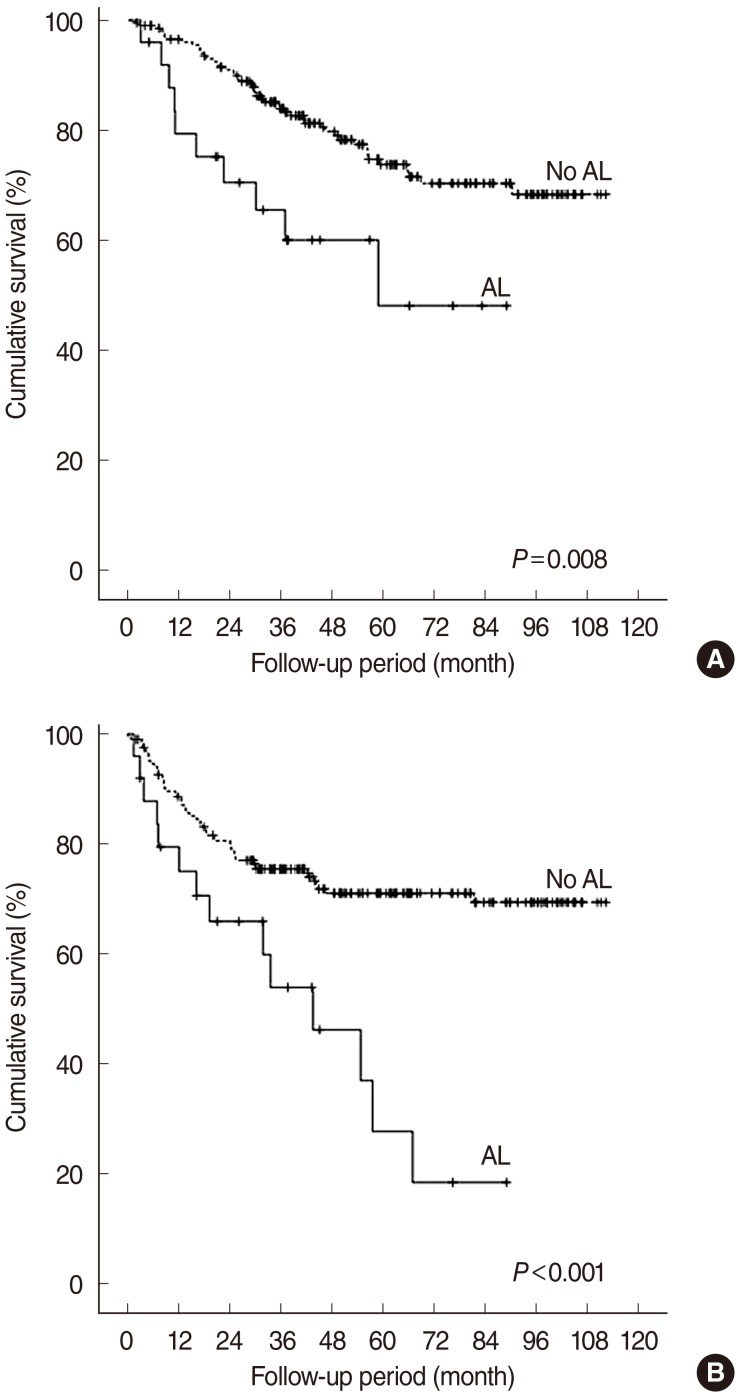
The median follow-up period was 64.7 months (range, 1.6 to 125.4 months). The LRC rates in the non-leakage and leakage groups were 78.4% and 62.9%, respectively (P=0.074). The OS (74.0% vs. 48.0%, P=0.008) and 5-year DFS (70.9% vs. 27.7%, P<0.001) were significantly different between the non-leakage and leakage groups (Fig. 1). Multivariate analysis showed, however, that AL was not an independent variable of LRC, DFS, or OS (P>0.1).
Postoperative wound infections are recognized as a leading long-term cause of decreased DFS in patients with HNSCC [12]. A clinical leakage rate of 10.8% was observed in this study, which is slightly lower than that reported in most previous studies involving conventional total laryngectomy and free-flap reconstruction for head and neck defects [1-9]. Fistulas usually become clinically apparent 7 to 11 days after surgery [6,7], with initial clinical signs of wound erythema, facial edema with soft tissue swelling, and fever. When AL further develops, surgical wound contamination by AL contents leads to wound dehiscence, skin necrosis, and soft tissue necrosis, resulting in severe morbidity, poor quality of life, and potential life-threatening sequalae [10]. Therefore, predicting and preventing AL is important.
Previous radiotherapy, prolonged operative time, poor nutritional status, delayed time from irradiation to surgery, extent of tumor and surgery, prophylactic antibiotics, radical neck dissection, delayed oral feeding, persistent carcinoma, flap reconstruction, infection, and blood transfusion have been reported as factors related to the development of AL in surgical patients with HNSCC [4-11,14]. In our study, multivariate logistic regression analysis revealed that salvage surgery and blood transfusion were independent predictors of AL. Our study also confirmed that AL is more likely to occur in patients who undergo salvage surgery, which is in agreement with previous studies [15-17]. Our results also suggest that salvage procedures in patients treated with concomitant chemoradiation are in general associated with a higher incidence of major wound complications. Patients who undergo salvage surgery may therefore be at a higher risk for developing AL [16]. Salvage surgery enhances the damaging effects of radiotherapy on normal tissues, leading to impaired wound healing and increased wound infection, dehiscence, and fistula formation. An association between blood transfusion, a marker of anemia, and adverse surgical outcome has been well-documented. In recent studies, postoperative hemoglobin levels lower than 12.5 g/dL were found to be significantly associated with an increased risk for the development of pharyngocutaneous fistula [6,18,19]. In addition, postoperative anemia is a consequence of high intraoperative blood loss and indicates the need for intraoperative transfusion, which significantly increased the incidence of AL from 7% to 28% in a previous report [20].
Preoperative recognition of a patient at risk may reduce the incidence of AL. In addition to salvage surgery and blood transfusion, we chose to analyze the effects of primary tumor site, perineural invasion, preoperative radiotherapy, and chemotherapy in the development of AL by multivariate analysis. Although our univariate analyses showed correlations between all of these factors and AL, no significance was found on multivariate analysis after accounting for the effect of the other factors. Previous reports are in disagreement with respect to the relationship between previous radiotherapy and fistula formation [21]. A meta-analysis, however, showed that preoperative radiotherapy increases the risk of fistula formation, and that fistula severity and duration were greater in patients with preoperative radiotherapy than in those without [11].
A previous study reported that the rate of tumor recurrence after total laryngectomy is not related to the presence of a fistula during the postoperative period [21]. The possible correlation between AL and tumor recurrence was not observed in our study. However, a tendency toward decreased 5-year DFS and OS in patients with AL was noted. To the best of our knowledge, this finding has not yet been reported. Although AL was not an independent factor affecting survival outcomes on multivariable analysis, our data suggest an important impact of AL on the prognostic outcome of patients following HNSCC surgery.
This study has potential limitations because of its retrospective nature. However, we analyzed the risk factors and prognostic values of AL in a large cohort of surgical HNSCC patients suspected of AL. Our results should be confirmed in a prospective study on a large number of patients.
In conclusion, we identified the risk factors for AL and the survival outcomes of HNSCC patients who develop AL. Patients who received salvage surgery and blood transfusion may require careful surveillance for the development of AL. AL patients were not associated with a significant increase in locoregional recurrences but showed a tendency toward decreased DFS and OS. We believe that our findings may help predict AL to facilitate proper management of surgical HNSCC patients.
References
1. Pelczar BT, Weed HG, Schuller DE, Young DC, Reilley TE. Identifying high-risk patients before head and neck oncologic surgery. Arch Otolaryngol Head Neck Surg. 1993; 8. 119(8):861–864. PMID: 8343249.

2. Panje WR, Namon AJ, Vokes E, Haraf DJ, Weichselbaum RR. Surgical management of the head and neck cancer patient following concomitant multimodality therapy. Laryngoscope. 1995; 1. 105(1):97–101. PMID: 7837923.
3. Mantravadi RV, Skolnik EM, Applebaum EL. Complications of postoperative and preoperative radiation therapy in head and neck cancers: a comparative study. Arch Otolaryngol. 1981; 11. 107(11):690–693. PMID: 7295163.

4. Bresson K, Rasmussen H, Rasmussen PA. Pharyngo-cutaneous fistulae in totally laryngectomized patients. J Laryngol Otol. 1974; 9. 88(9):835–842. PMID: 4214887.

5. Johansen LV, Overgaard J, Elbrond O. Pharyngo-cutaneous fistulae after laryngectomy: influence of previous radiotherapy and prophylactic metronidazole. Cancer. 1988; 2. 61(4):673–678. PMID: 3338032.
6. Redaelli de Zinis LO, Ferrari L, Tomenzoli D, Premoli G, Parrinello G, Nicolai P. Postlaryngectomy pharyngocutaneous fistula: incidence, predisposing factors, and therapy. Head Neck. 1999; 3. 21(2):131–138. PMID: 10091981.

7. Mäkitie AA, Irish J, Gullane PJ. Pharyngocutaneous fistula. Curr Opin Otolaryngol Head Neck Surg. 2003; 4. 11(2):78–84. PMID: 14515083.

8. Joo YH, Sun DI, Park JO, Cho KJ, Kim MS. Factors predicting fistula following radial forearm free flap reconstruction for head and neck cancer. Oral Oncol. 2010; 9. 46(9):684–687. PMID: 20729137.

9. Andrades P, Pehler SF, Baranano CF, Magnuson JS, Carroll WR, Rosenthal EL. Fistula analysis after radial forearm free flap reconstruction of hypopharyngeal defects. Laryngoscope. 2008; 7. 118(7):1157–1163. PMID: 18438265.

10. Boscolo-Rizzo P, De Cillis G, Marchiori C, Carpene S, Da Mosto MC. Multivariate analysis of risk factors for pharyngocutaneous fistula after total laryngectomy. Eur Arch Otorhinolaryngol. 2008; 8. 265(8):929–936. PMID: 18247039.

11. Paydarfar JA, Birkmeyer NJ. Complications in head and neck surgery: a meta-analysis of postlaryngectomy pharyngocutaneous fistula. Arch Otolaryngol Head Neck Surg. 2006; 1. 132(1):67–72. PMID: 16415432.
12. Grandis JR, Snyderman CH, Johnson JT, Yu VL, D'Amico F. Postoperative wound infection: a poor prognostic sign for patients with head and neck cancer. Cancer. 1992; 10. 70(8):2166–2170. PMID: 1394047.

13. Aarts MC, Rovers MM, Grau C, Grolman W, van der Heijden GJ. Salvage laryngectomy after primary radiotherapy: what are prognostic factors for the development of pharyngocutaneous fistulae? Otolaryngol Head Neck Surg. 2011; 1. 144(1):5–9. PMID: 21493379.
14. Greene FL, Page DL, Fleming ID. American Joint Committee on Cancer (AJCC) cancer staging manual. 6th ed. New York: Springer;2002.
15. Ganly I, Patel S, Matsuo J, Singh B, Kraus D, Boyle J, et al. Postoperative complications of salvage total laryngectomy. Cancer. 2005; 5. 103(10):2073–2081. PMID: 15816049.

16. Tsou YA, Hua CH, Lin MH, Tseng HC, Tsai MH, Shaha A. Comparison of pharyngocutaneous fistula between patients followed by primary laryngopharyngectomy and salvage laryngopharyngectomy for advanced hypopharyngeal cancer. Head Neck. 2010; 11. 32(11):1494–1500. PMID: 20187019.

17. Sassler AM, Esclamado RM, Wolf GT. Surgery after organ preservation therapy: analysis of wound complications. Arch Otolaryngol Head Neck Surg. 1995; 2. 121(2):162–165. PMID: 7840923.

18. Cavalot AL, Gervasio CF, Nazionale G, Albera R, Bussi M, Staffieri A, et al. Pharyngocutaneous fistula as a complication of total laryngectomy: review of the literature and analysis of case records. Otolaryngol Head Neck Surg. 2000; 11. 123(5):587–592. PMID: 11077346.

19. Celikkanat S, Koc C, Akyol MU, Ozdem C. Effect of blood transfusion on tumor recurrence and postoperative pharyngocutaneous fistula formation in patients subjected to total laryngectomy. Acta Otolaryngol. 1995; 7. 115(4):566–568. PMID: 7572137.
20. Hier M, Black MJ, Lafond G. Pharyngo-cutaneous fistulas after total laryngectomy: incidence, etiology and outcome analysis. J Otolaryngol. 1993; 6. 22(3):164–166. PMID: 8371326.
21. Markou KD, Vlachtsis KC, Nikolaou AC, Petridis DG, Kouloulas AI, Daniilidis IC. Incidence and predisposing factors of pharyngocutaneous fistula formation after total laryngectomy: is there a relationship with tumor recurrence? Eur Arch Otorhinolaryngol. 2004; 2. 261(2):61–67. PMID: 12856129.

Fig. 1
Overall (A) and disease-free (B) survivals according to the presence of anastomotic leakage (AL). The log-rank test, P<0.05.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download