Abstract
Objectives
One of the antidiabetic drugs, metformin, have shown that it prevented oxidative stress-induced death in several cell types through a mechanism involving the opening of the permeability transition pore and cytochrome c release. Thus, it is possible that the antioxidative effect of metformin can also serve as protection against gentamicin-induced cytotoxicity related to reactive oxygen species (ROS). The aim of this study was to examine the protective effect of metformin on gentamicin-induced vestibulotoxicity in primary cell culture derived from rat utricle.
Methods
For vestibular primary cell culture, rat utricles were dissected and incubated. Gentamicin-induced cytotoxicity was measured in both the auditory and vestibular cells. To examine the effects of metformin on gentamicin-induced cytotoxicity in the primary cell culture, the cells were pretreated with metformin at a concentration of 1 mM for 24 hours, and then exposed to 2.5 mM gentamicin for 48 hours. The intracellular ROS level was measured using a fluorescent dye, and also measured using a FACScan flow cytometer. Intracellular calcium levels in the vestibular cells were measured with calcium imaging using Fura-2 AM.
Results
Vestibular cells were more sensitive to gentamicin-induced cytotoxicity than auditory hair cells. Metformin protects against gentamicin-induced cytotoxicity in vestibular cells. Metformin significantly reduced a gentamicin-induced increase in ROS, and also reduced an increase in intracellular calcium concentrations in gentamicin-induced cytotoxicity.
Ménière's disease is a chronic condition that affects a substantial number of patients each year all over the world. The disease is characterized by paroxysmal episodes of vertigo lasting more than 20 minutes, with fluctuating sensorineural hearing loss, tinnitus, and ear fullness. Although no definite cure is currently available for Ménière's disease, more than 85% of the patients are treated either by changes in lifestyle and medical treatment or by minimally invasive surgical procedures, such as intratympanic gentamicin therapies and endolymphatic sac surgery [1,2]. An intratympanic injection of gentamicin seems to be an effective treatment for vertigo in intractable Ménière's disease, but it is associated with the risk of hearing loss, which was reported in about 30% of patients [3,4,5,6].
The reason for using gentamicin for treating Ménière's disease is that it causes selective damage to vestibular dark cells. Rather than destroying the auditory hair cells, gentamicin selectively destroys the endolymph secreting vestibular dark cells. The reduction in endolymph secreted by the dark cells helps relieve the symptoms of the disease [7,8,9,10].
Gentamicin is believed to initiate the intrinsic apoptotic pathway by condensation of the nuclei of the hair cells, followed by the loss of the mitochondrial membrane potential and, finally, apoptosis [11]. Gentamicin seems to enhance the formation of reactive oxygen species (ROS), which induce the opening of the mitochondrial permeability transition (MPT) pore [11,12]. The MPT pore is involved in the intrinsic apoptotic pathway via the release of cytochrome c into cytosol, and pore is opened resulting in a swelling of mitochondria and destruction of the outer mitochondrial membrane [13]. The presence of ROS and resulting apoptosis can lead to increased intracellular calcium concentrations [14,15,16]. One of the studies, performed using tissue cultures from chick auditory sensory epithelium, showed that gentamicin caused a dose-dependent increase in intracellular calcium levels in the hair cells [17]. It is hypothesized that the mechanism of hair-cell death caused by gentamicin is related to an increase in the intracellular calcium concentration [18].
Recent investigations have shown that metformin prevented oxidative stress-induced death in several cell types [19,20]. The production of hyperglycemia-induced mitochondrial ROS plays a role in the development of diabetic complications including atherosclerosis, and metformin inhibits ROS by stimulating AMP-activated protein kinase activity [21]. Metformin significantly reduced intracellular ROS levels and increased the expression of thioredoxin, a protein with antioxidant properties [22]. Another study showed that metformin attenuated ROS production and the associated DNA damage and mutations [23].
The aim of this study was to examine whether metformin protected against vestibulotoxicity in rat primary cell culture after exposure to gentamicin.
Sprague-Dawley rats aged from 7 to 10 days were obtained and maintained in the Animal Care Facility. All experiments were approved by the University Institutional Animal Care and Use Committee. Animals were anesthetized using an inhalant anesthetic (Isoflurane; MINRAD Inc., Bethlehem, PA, USA) and killed by decapitation.
The utricles were dissected using the sterile technique for the primary cell culture and were cultured in 6-well tissue culture plates (5-6 utricles per well). Otoconia were gently removed from the utricles with a stream of phosphate buffered saline (PBS) applied via a 28 G needle and syringe. Subsequently, the utricles were gently opened and fixed at the bottom of culture plates (Fig. 1). The culture medium consisted of the Dulbecco's Modified Eagle's Medium (DMEM; Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco BRL), N-1 supplement (Sigma, St. Louis, MO, USA), and 100 U/mL penicillin (Sigma).
Establishment of the HEI-OC1 cell line was facilitated by the development of a transgenic immortomouse. A cochlear explant cultured for 28 days under permissive conditions (33℃) and later moved to 39℃ and cultured for an additional 30-day period. Monolayer of cells was growing in the periphery beyond the ridge between the multilayered regions of the original explant. The cells in the monolayer, at the boundary with the original explant, display a very homogeneous phenotype. The HEI-OC1 line was derived from these cells. HEI-OC1 cells were maintained in DMEM containing 10% FBS and 50 U/mL interferon-γ (PeproTech, USA) without antibiotics at 33℃ under 10% CO2 in air. HEI-OC1 cells express several molecular markers that are characteristic of the organ of Corti sensory cells, and are very sensitive to ototoxic drugs.
Gentamicin (Sigma) was prepared as a 2.5 mM solution in the cell culture media and added directly to the culture well. No gentamicin was added to control cultures. 1,1-dimethylbiguanide hydrochloride (Metformin; Sigma) was used at the concentration of 1 mM and added for cellular protection. The utricles were incubated for 48 hours at 37℃ in a 5% CO2 and 95% air environment.
The uptake and conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) to crystals of dark violet formazan depends on cell viability. The vestibular cells (4×104 cells/well of 24-well plate) were incubated with various concentrations of gentamicin to determine the half maximal inhibitory concentration (IC50; the concentration that is required for 50% inhibition) for 48 hours, and the effects of gentamicin were measured using the MTT assay. To examine the effects of metformin on gentamicin-induced cytotoxicity in the primary cell culture, the cells were pretreated with metformin at a concentration of 1 mM for 24 hours, and then exposed to gentamicin (2.5 mM, 48 hours). For the MTT assay, 200 µL of the MTT solution (5 mg/mL) was added to the cell, and the plates were then incubated for 3 hours at 37℃ in a 5% CO2 and 95% air. The insoluble formazan crystals were centrifuged, and the pellets were dissolved by the addition of dimethyl sulfoxide (DMSO; 500 µL/well). The optical density was measured using a microplate reader at 540 nm (Spectra Max; Molecular Devices, Sunnyvale, CA, USA). Results were from 5 separate experiments in triplicate [24,25,26].
The intracellular ROS level was measured using a fluorescent dye, 2',7'-dichlorofluorescein diacetate (DCFH-DA, Sigma). In the presence of an oxidant, DCFH was converted into highly fluorescent 2',7'-dichlorofluorescein (DCF). For the assay, the vestibular cells were cultured on the cover slips and then treated with 2.5 mM of gentamicin for 48 hours in the presence or absence of 1 mM metformin. The cells were washed twice with a serum-free medium without phenol red and incubated with 5 µM DCFH-DA for 15 minutes at 37℃. After 3 washings, the cells were fixed with 3.7% glutaraldehyde for 10 minutes at room temperature. They were then incubated with 10 µg/mL Hoechst 33258 (Sigma) for 20 minutes at room temperature in the dark. After washing twice with PBS, the samples were immediately observed at 387-nm excitation, and then the fluorescent intensity was measured at 485-nm excitation and 538-nm emission by a long-term real-time live-cell image system (Lambda DG-4; Sutter Int., Novato, CA, USA). H2O2 at a concentration of 100 µM was used as positive control.
Intracellular ROS levels were also measured using a FACScan flow cytometer (BD Biosciences, Heidelberg, Germany) at an excitation wavelength of 495 nm and an emission wavelength of 530 nm (10,000 cells/sample). The mean fluorescence intensities were obtained by histogram statistics by using the BD FACSDiva software (BD Biosciences), and analyzed by previously described methods (results from 5 separate experiments in triplicate) [24,25,26].
Intracellular calcium levels in the vestibular cells were measured with Fura-2 AM (8 µM) in L-15 media, with digital microscopy (Universal; Carl Zeiss Inc., Thornwood, NY, USA). Fura-2 is a ratiometric dye that can report the calcium concentration independent of the dye concentration or image thickness. A field of cells was monitored by sequential dual excitation at 352 and 380 nm, and the image ratio was analyzed by a previously described method [27,28]. Images were acquired every 3 seconds, and calcium concentrations for negative control (baseline) were measured in the first 10 cycles (30 seconds).
The cells on the coverslip were exposed to gentamicin between cycle 30 (90 seconds) and cycle 150 (450 seconds). The response to gentamicin was measured from the time of gentamicin application to the application of ionomycin (positive control) at cycle 150. Changes in intracellular calcium concentrations were calculated using 10 cycles within the final one-third of measurements in the presence of gentamicin. The baseline response was compared to this mean calcium indicator response. After gentamicin exposure, the cells were finally exposed to 1 µM of ionophore ionomycin from cycle 150 to cycle 180 (30 cycles; 90 seconds). An increase in the calcium concentration after ionomycin exposure was taken as the maximal response. Each set of images for calcium measurement also included a bright field image of the field of cells under study. Twenties of the cells that had an average diameter (long and short axis) of over 15 µM were analyzed. Calcium imaging was performed in 3 groups: control group (no use of gentamicin); gentamicin group (commercial concentration of 83.7 mM gentamicin); and gentamicin-plus-metformin group (gentamicin application in the presence of 1 mM metformin). All experiments were performed in triplicate. The ratio of the change in the calcium concentration was analyzed in all groups by using the analysis of variance (ANOVA).
As shown in Fig. 2, gentamicin decreased the viability of cells in time- and dose-dependent manners. In the HEI-OC1 cell line, cells treated with 1, 2, 3, 4, and 5 mM gentamicin for 48 hours showed the viabilities of 93.2%, 91.4%, 63.1%, 45.6%, and 5.7%, respectively (Fig. 2A, n=5). Thus, gentamicin at a concentration of 4 mM applied for 48 hours was chosen as an adequate experimental condition for the IC50. In the vestibular cell culture, cells were treated with 1, 1.5, 2, 2.5, 3, 3.5, and 4 mM gentamicin for 48 hours. As shown in Fig. 2B, the viabilities were 83.7%, 77.3%, 69.4%, 57.3%, 40.9%, 33%, and 26.9%, respectively (n=5). Thus, gentamicin at a concentration of 2.5 mM applied for 48 hours was chosen as an adequate experimental condition for the IC50 of the primary cell culture.
The higher the concentration of gentamicin applied, the more cells were damaged in both cell cultures. The comparison in the ratio of relative viability between the auditory cell line and vestibular primary cell culture is shown in Fig. 2C (y=-0.1x+1.1). A trend line showed a steady ratio between the auditory cell line and vestibular primary cells, and vestibular cells were more sensitive to gentamicin-induced cytotoxicity (Fig. 1C).
In the negative-control group, metformin promoted slight cellular growth, showing a maximal cell viability of 111.8% at the concentration of 1 mM metformin (nonsignificant compared to the control group; P>0.05) (Fig. 3). Metformin provided significant protection (59.9%±3.7% for the gentamicin group vs. 75.6%±5% for the gentamicin-plus-metformin group, mean±SE, n=5) against the toxic effect of gentamicin at a concentration of 2.5 mM applied for 48 hours (Fig. 3).
Gentamicin-induced vestibulotoxicity and the protective effect of metformin were also verified by microscopic anatomical examination. In the control group, normal vestibular cells were well-cultured and spindle-shaped (Fig. 4A). In the group treated with 1 mM metformin, metformin promoted slight cellular growth (111.8%), similar to that observed in the control group (Fig. 4B). In the group treated with 2.5 mM gentamicin, numerous necrotic whitish cell bodies floated on the surface of the cell culture plates. Furthermore, the remaining cells showed condensation and shrinkage with nuclear fragmentation (Fig. 4C). Compared to the gentamicin group, in the group treated with gentamicin and metformin, the cell size was within the normal range and necrotic bodies were quite reduced. However, nuclear fragmentation and necrosis were still more frequently observed than in the control group (Fig. 4D). Thus, microscopic appearances showed a protective effect of metformin on gentamicin-induced cytotoxicity in the vestibular primary cell culture, reflecting similar results obtained by the cell viability assay.
Compared to the control group, both the gentamicin and H2O2 groups showed significant ROS generation (148%±12.3% and 180%±22.4%, respectively). However, metformin significantly protected the vestibular cells from gentamicin-induced cytotoxicity, and ROS production in the gentamicin-plus-metformin group (120%±11.7%) was significantly lower than that in the gentamicin and H2O2 groups (Fig. 5). ROS production was not significantly different between the control (100%), metformin (109%±8.2%), and gentamicin-plus-metformin groups (120%±11.7%).
In the control group (saline application), little changes in calcium levels were observed in the vestibular cells, and response to ionomycin was normal (Fig. 6A). In the gentamicin group, gentamicin induced an abrupt increase in intracellular calcium concentrations in all tested cells, comparable to the level of maximal response to ionomycin (Fig. 6B). In the gentamicin-plus-metformin group, 2 cells showed an abrupt increase in calcium levels followed by a return to baseline levels (arrow), in contrast to other cells that showed a slight and stable increase in calcium levels (Fig. 6C). In the control group, no significant increase or decrease in intracellular calcium levels by 97%±6% was observed. After the application of gentamicin, the ratio of changes increased by 150%±18%. However, metformin significantly reduced intracellular calcium levels in gentamicin-induced cytotoxicity by 108%±14%. The ratio of changes in calcium levels did not differ between the control and gentamicin-plus-metformin groups (Fig. 6D).
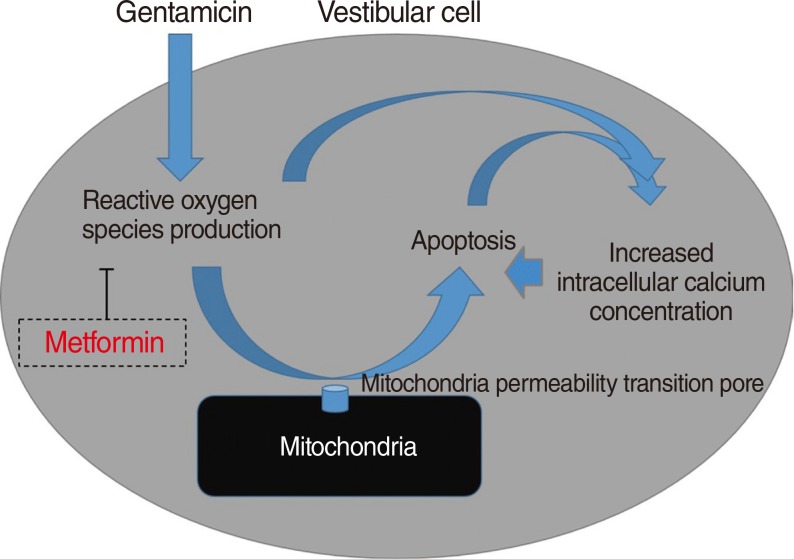
This study is one of the first to investigate the protective effects of metformin on gentamicin-induced cytotoxicity [25]. In the present study, we showed that metformin significantly reduced a gentamicin-induced increase in ROS and inhibited apoptotic cell changes. We believe that the inhibitory effect of metformin on ROS production is the main mechanism of protection against gentamicin-induced vestibulotoxicity (Fig. 7).
Anti-ROS effect of metformin has been reported. For example, it was shown to decrease intracellular production of ROS in aortic endothelial cells by inhibiting the formation of ROS produced mainly by NADPH oxidase [29]. Metformin was also reported to inhibit the generation of ROS by endothelial and smooth muscle cells [30]. Moreover, it was shown to reduce intracellular ROS levels through expression of thioredoxin and protection against DNA damage [22,23]. Metformin decrease excess hepatic gluconeogenesis without raising insulin levels, it rarely leads to significant hyper/hypoglycemia. Presumably metformin use for anti-ROS or protective effect is no significant risk in healthy person.
In this study, the vestibular cells were more sensitive to gentamicin-induced cytotoxicity than the auditory hair cells. For example, gentamicin at a concentration of 2.5 mM (IC50) applied to the auditory and vestibular cells induced different amount of apoptosis, and viability was 57.3% and 78%, respectively (correspond to 50% vs. 72%). Only a 73% dose of gentamicin used in the auditory hair cells was needed to induce the same level of apoptosis in the vestibular cells. At the calibrated dose of gentamicin for vertigo control (IC50), cochlear cell damage was 28%, which was similar to the risk of hearing loss, about 30%, observed on the clinical application of gentamicin to treat Meniere's disease [4,5,6,7,8]. When gentamicin was instilled into the middle ear, vestibular cells were shown to be more vulnerable than the cochlear auditory cells. Thus, the present study can serve as in-vitro evidence of selective vulnerability to gentamicin in the inner ear system.
Increased intracellular calcium levels and excessive ROS production were important factors in the apoptosis of gentamicin-induced cytotoxicity, as shown in the previous studies [27]. Thus, the protective mechanism of metformin in gentamicin-induced cytotoxicity involves both the inhibition of excessive ROS production and stabilization of the intracellular calcium concentration.
In this study, the growth of vestibular primary cell culture was reduced over time. After dissection and cell seeding, the cell culture dish was fully filled with vestibular cells in about 2 weeks. At the beginning, cell doubling could be observed within 4 days, but the growth rate was reduced by about 13% with each doubling time. Thus, experiments in primary cell culture should be performed during the several initial phases of cell growth. Owing to unsteady growth rate in vestibular primary cell culture, the variability in the results of the cell viability test is wider than that observed for the artificial auditory cell line (HEI-OC1).
The limitation of this study is comparing vestibular primary cell culture and artificial cochlear cell line, which growth rate may be different. Furthermore, repeated experiment may be difficult because of slow growth rate of vestibular cell. An ideal study design might involve a comparison between cochlear and vestibular cells in the same inner ear of a rat with systemic gentamicin-induced toxicity. However, the cochlear cells in a 7- to 10-day-old rat are not yet mature and fully functional. In an adult rat, on the other hand, dissection of the cochlea is difficult, thus organotypic culture and toxicity study cannot be easily performed. For this reason, we compared artificial auditory cells and vestibular primary cells.
In summary, metformin significantly reduced a gentamicin-induced increase in ROS, inhibited gentamicin-induced apoptosis, and stabilized the intracellular calcium concentration. Our study suggests that the inhibitory effect of metformin on ROS production is the main mechanism of protection against gentamicin-induced vestibulotoxicity.
ACKNOWLEDGMENTS
This study was supported by the Korea University Grant (K1220291) and the National Research Foundation of Korea Grant funded by the Korean Government Ministry of Education, Science and Technology (MEST), Basic Research Promotion Fund (NRF-2010-013-E00015). These funding sources provide only financial support and play no specific scientific role in this study.
References
2. Schuknecht HF. Ablation therapy in the management of Meniere's disease. Acta Otolaryngol Suppl. 1957; 132:1–42. PMID: 13457879.
3. Pullens B, van Benthem PP. Intratympanic gentamicin for Meniere's disease or syndrome. Cochrane Database Syst Rev. 2011; 3. (3):CD008234. PMID: 21412917.
4. Huon LK, Fang TY, Wang PC. Outcomes of intratympanic gentamicin injection to treat Meniere's disease. Otol Neurotol. 2012; 7. 33(5):706–714. PMID: 22699980.
5. Blakley BW. Update on intratympanic gentamicin for Meniere's disease. Laryngoscope. 2000; 2. 110(2):236–240. PMID: 10680922.

6. Minor LB. Intratympanic gentamicin for control of vertigo in Meniere's disease: vestibular signs that specify completion of therapy. Am J Otol. 1999; 3. 20(2):209–219. PMID: 10100525.
7. Pender DJ. Gentamicin tympanoclysis: effects on the vestibular secretory cells. Am J Otolaryngol. 1985; Sep-Oct. 6(5):358–367. PMID: 3907388.

8. Beck C. Intratympanic application of gentamicin for treatment of Meniere's disease. Keio J Med. 1986; 3. 35(1):36–41. PMID: 3747323.

9. Tange RA, Conijn EA, van Zeijl LG, Huizing EH. Pattern of gentamicin-induced cochlear degeneration in the guinea pig: a morphological and electrophysiological study. Arch Otorhinolaryngol. 1982; 236(2):173–184. PMID: 7150082.
10. Quaranta A, Scaringi A, Aloidi A, Quaranta N, Salonna I. Intratympanic therapy for Meniere's disease: effect of administration of low concentration of gentamicin. Acta Otolaryngol. 2001; 4. 121(3):387–392. PMID: 11425206.
11. Dehne N, Rauen U, de Groot H, Lautermann J. Involvement of the mitochondrial permeability transition in gentamicin ototoxicity. Hear Res. 2002; 7. 169(1-2):47–55. PMID: 12121739.

12. Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G. Mitochondrial membrane permeabilization during the apoptotic process. Ann N Y Acad Sci. 1999; 887:18–30. PMID: 10668461.

13. Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death: mechanistic aspects and methodological issues. Eur J Biochem. 1999; 9. 264(3):687–701. PMID: 10491114.

14. Brustovetsky N, Brustovetsky T, Purl KJ, Capano M, Crompton M, Dubinsky JM. Increased susceptibility of striatal mitochondria to calcium-induced permeability transition. J Neurosci. 2003; 6. 23(12):4858–4867. PMID: 12832508.

15. Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006; 5. 273(10):2077–2099. PMID: 16649987.

16. Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria: modulation by electron flow through the respiratory chain complex I. J Biol Chem. 1998; 5. 273(20):12662–12668. PMID: 9575229.
17. Hirose K, Westrum LE, Stone JS, Zirpel L, Rubel EW. Dynamic studies of ototoxicity in mature avian auditory epithelium. Ann N Y Acad Sci. 1999; 11. 884:389–409. PMID: 10842609.

18. Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002; 2. 164(1-2):115–126. PMID: 11950531.
19. El-Mir MY, Detaille D, R-Villanueva G, Delgado-Esteban M, Guigas B, Attia S, et al. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J Mol Neurosci. 2008; 34(1):77–87. PMID: 18040888.

20. Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, et al. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004; 9. 382(Pt 3):877–884. PMID: 15175014.

21. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, Yano M, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006; 1. 55(1):120–127. PMID: 16380484.

22. Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, et al. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010; 5. 396(2):199–205. PMID: 20398632.

23. Algire C, Moiseeva O, Deschenes-Simard X, Amrein L, Petruccelli L, Birman E, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res (Phila). 012; 4. 5(4):536–543. PMID: 22262811.

24. Im GJ, Chang JW, Choi J, Chae SW, Ko EJ, Jung HH. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother Res. 2010; 4. 24(4):614–621. PMID: 20020438.

25. Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res. 2011; 12. 282(1-2):92–96. PMID: 21979311.
26. Choi J, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol. 2013; 2. 33(2):125–133. PMID: 22147442.

27. Chang J, Yang JY, Choi J, Jung HH, Im GJ. Calcium imaging in gentamicin ototoxicity: increased intracellular calcium relates to oxidative stress and late apoptosis. Int J Pediatr Otorhinolaryngol. 2011; 12. 75(12):1616–1622. PMID: 22015113.

28. Orrenius S, McConkey DJ, Nicotera P. Role of calcium in toxic and programmed cell death. Adv Exp Med Bio. 1991; 283:419–425. PMID: 1648868.

29. Ouslimani N, Peynet J, Bonnefont-Rousselot D, Therond P, Legrand A, Beaudeux JL. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005; 6. 54(6):829–834. PMID: 15931622.

30. Bellin C, de Wiza DH, Wiernsperger NF, Rosen P. Generation of reactive oxygen species by endothelial and smooth muscle cells: influence of hyperglycemia and metformin. Horm Metab Res. 2006; 11. 38(11):732–739. PMID: 17111300.

Fig. 1
Vestibular primary cell culture. (A) Rat utricles were removed for primary cell culture. Otoconia were gently removed from the utricles and the utricles were gently opened and fixed at the bottom of culture plates. Spreading vestibular cells (arrows) from the utricles were observed after 2 days of incubation. (B-D) Vestibular cells were spindle-shaped, and spreading to whole culture plate. Magnifications were ×40, ×40, ×100, and ×200, respectively.

Fig. 2
Comparison of gentamicin (GM)-induced cytotoxicity between the auditory (HEI-OC1) cell line and vestibular primary cell culture. (A) GM-induced cytotoxicity in the auditory cell line. Cell viability was 45.5% at the GM concentration of 4 mM for 48 hours. (B) GM-induced cytotoxicity in the primary cell culture. Cell viability was 57.3% at the GM concentration of 2.5 mM for 48 hours. (C) Ratio of relative viability between the auditory cell line and primary cell culture. The higher the concentration of GM was applied to cell cultures, the more cells were damaged. A trend line showed a steady ratio between the auditory cell line and primary cell culture, and vestibular cells were more sensitive to GM-induced cytotoxicity. Results from 5 separate experiments in triplicate.

Fig. 3
Protective effect of metformin measured by the MTT assay. In the group receiving 1 mM metformin, metformin promoted slight cellular growth, showing maximal cell viability of 111.8% at the concentration of 1 mM metformin (no significant compared with control, P>0.05). Metformin provided significant protection (59.9%±3.7% for the gentamicin [GM] group vs. 75.6%±5% for the GM-plus-metformin group, mean±SE, n=5) against the toxic effect of 2.5 mM GM applied for 48 hours (*P<0.05, compared with the GM group). Results from 5 separate experiments in triplicate.
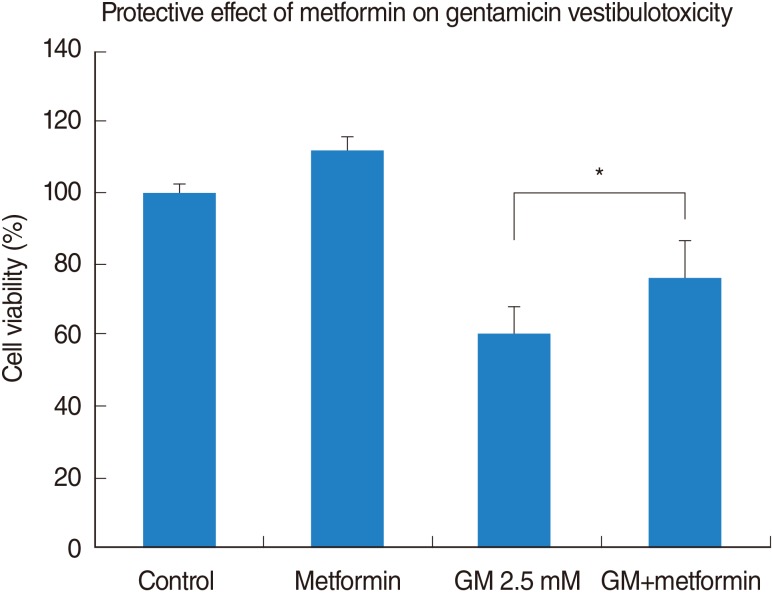
Fig. 4
Representative microscopic appearance: protective effect of metformin on gentamicin-induced cytotoxicity in vestibular primary cell culture. (A) Control group, normal vestibular primary cell culture. (B) Group receiving 1 mM metformin. Metformin promoted slight cellular growth, showing similar appearance with the control group. (C) Group receiving 2.5 mM gentamicin. Many necrotic whitish cell bodies floated on the surface of cell culture plates. The remaining cells showed condensation and shrinkage with nuclear fragmentation. (D) Gentamicin-plus-metformin group. Metformin protected against gentamicin-induced cytotoxicity. Compared with the gentamicin group, the cell size was within the normal range and necrotic bodies were fewer. However, more cells with nuclear fragmentation and necrosis were observed compared with the control group.

Fig. 5
Measurement of intracellular reactive oxygen species (ROS) production (DCFH-DA, green). (A) Control, (B) gentamicin (GM), (C) GM plus metformin, (D) vestibular cells were treated with 2.5 mM GM in the presence or absence of 1 mM metformin for 48 hours. Metformin significantly protected vestibular cells from GM-induced cytotoxicity, and ROS production in the GM-plus-metformin group was significantly lower than that in the GM and H2O2 groups (**P<0.01, ***P<0.001, respectively). However, ROS production was not significantly different between the control, metformin, an GM-plus-metformin groups (control, vestibular cells in control media; GM, cells treated with 2.5 mM GM for 48 hours; metformin, cells treated with 1 mM metformin; GM plus metformin, cells treated with 2.5 mM GM and 1 mM metformin for 48 hours; H2O2, 100 µM H2O2 was used as positive control). Results from 5 separate experiments in triplicate.

Fig. 6
Representative data of calcium imaging shows protective effects on gentamicin (GM)-induced cytotoxicity. (A) In control, no changes of calcium concentration were observed in vestibular cells. Responses to ionomycin were normal. (B) Direct application of commercial GM at concentration of 83.7 mM induced an abrupt increase in intracellular calcium concentrations in all cells tested, comparable to the level of maximal response to ionomycin. (C) In the GM-plus-metformin group, 2 cells showed an abrupt increase in calcium concentrations followed by a return to baseline levels (arrows), in contrast to other cells that had a slight and stable increase in calcium levels. (D) A before-and-after comparison shows that an increase in intracellular calcium levels was observed in the GM group compared with control (**P<0.01), but metformin reduced intracellular calcium elevation in GM-induced cytotoxicity (*P<0.05). The ratio of change in the calcium concentration was not different between the control and GM-plus-metformin groups. Results from 3 separate experiments, each experiment consisting of 20 cells.
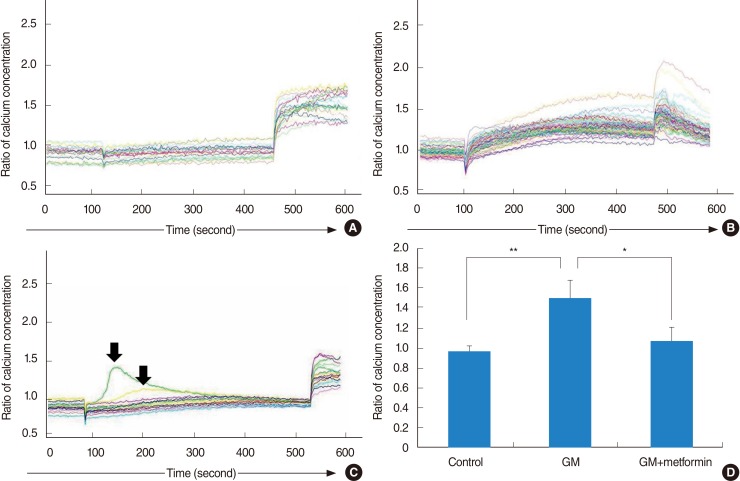
Fig. 7
Schematic mechanism on gentamicin-induced cytotoxicity. Gentamicin may enhance the formation of reactive oxygen species (ROS), which induce the opening of the mitochondrial permeability transition (MPT) pore. The MPT pore is involved in the intrinsic apoptotic pathway, and pore is opened resulting in a swelling of mitochondria and destruction of the outer mitochondrial membrane. The presence of ROS and resulting apoptosis can lead to increased intracellular calcium concentrations. Metformin significantly reduced a gentamicin-induced increase in ROS, inhibited gentamicin-induced apoptosis, and stabilized the intracellular calcium concentration.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download