Abstract
Objectives
Utilisation of high-frequency drills is known to increase noise induced hearing loss due to increasing the damages of inner ear cells. This study aimed to investigate whether preconditioning by using dexmedetomidine (DEX) decreased the occurrence of ischemia in inner cells of the ear.
Methods
We utilised a transgenic zebrafish line Brn3C, and the embryos were collected from breeding adult zebrafish. Five-day-old larvae were cultured at the density of 50 embryos, and the larvae were classified into 4 groups: control, cisplatin group, DEX group, and DEX+yohimbine; adrenoreceptor blocker group. The DEX group was categorised into 3 subgroups by dosage; 0.1, 1, and 10 µM. Preconditioning was performed for 150 minutes and then exposed to cisplatin for 6 hours. The experiment was performed in 7 replicates for each group and the number of hair cells in 3 parts of the neuromasts of each fish was determined.
The recent improvements in medical techniques have resulted in the use of high-frequency drills. Although the use high-frequency drills has made surgical procedures more convenient and safer, they generate noise vibrations that damage the cells of the inner ear (hair cells) during mastoidectomy, thereby causing hearing loss in 1.2%-4.5% of patients [1]. Farzanegan et al. [2] reported that hearing loss developed after craniotomy when high-frequency drills were used at speeds within the range of 4,000-6,000 Hz.
The cause of hearing loss involves the insufficiency of oxygen in hair cells due to vibration, which is because of an increased production of the reactive oxygen species (ROS) during ischaemia, resulting in the apoptosis of hair cells. Hong et al. [3] showed that the utilisation of inhibitory drugs such as edaravone reduced hair cell damage and Choi et al. [4] reported that substances extracted from vanilla such as apocynin imparted protective effects on neurons. However, because these drugs are not commonly used in head and neck surgery, their clinical importance is not clear.
Dexmedetomidine (DEX), an alpha-2 adrenoreceptor selective agonist, is a novel anaesthetic agent that is currently being investigated for its clinical applications. When DEX is administered prior to the development of ischaemia, preconditioning effects such as inhibition of ROS hyperactivity through adreno-receptors were observed [5,6,7,8]. ROS hyperactivity is a critical causative factor of noise-induced hearing loss, and therefore, the authors hypothesised that the application of DEX would decrease hair cell damage by imparting preconditioning effects, similar to that observed between ischaemia and cisplatin in a zebrafish model.
The transgenic zebrafish line Brn3C: enhanced green fluorescent protein (EGFP), which emits green luminescence in neuromasts, was utilised in this study. Zebrafish embryos were collected by breeding adult zebrafish at 28.5℃ in the laboratory. The embryos were cultured at a density of 50 embryos per 100-mm2 Petri dish. The composition of the embryo culture medium was as follows: 1 mM MgSO4, 120 mM KH2PO4, 74 mM Na2HPO4, 1 mM CaCl2, 500 mM KCl, 15 mM NaCl, and 500 mM NaHCO3 in dH2O. DEX was provided by Hospira Inc. (Lake Forest, IL, USA) and the other reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
The experiment was carried out using 5-day-old larvae, which were classified into 4 groups: control, cisplatin group, DEX preconditioning group, and DEX and yohimbine preconditioning group. Preconditioning was performed for 150 minutes, and exposure to 1 mM of cisplatin [4] was conducted for 6 hours; the control group, which did not receive any treatment, was monitored for 8 hours. After the cisplatin treatment, the medium was removed and the dish was washed three times with phosphate buffer solution (PBS), followed by quantification of hair cells in the neuromasts. Preconditioning was conducted by dividing the larvae into 3 subgroups and treating each group with a different DEX concentration (0.1, 1, and 10 µM) for 150 minutes. After treatment, the dishes were washed three times with PBS and changed to the medium containing cisplatin (Fig. 1). Yohimbine, a selective adrenoreceptor blocker, at a reference concentration of 100 µM, was injected with 10 µM DEX during preconditioning to determine whether the effects of DEX were reversible. The experiment was performed using 7 replicates for each group and 3 neuromasts were measured per fish.
To determine the expression of the adrenoreceptors in zebrafish hair cells, immunostaining was performed using adrenoreceptor-specific antibodies. At 5 days post-fertilisation (dpf), the transgenic zebrafish larvae were fixed in 4% paraformaldehyde solution at 4℃ for 12 hours. The fixed embryos were washed three times with PBS for 10 minutes each. These were then treated with acetone and 0.25% trypsin/PBS, washed with PBS buffer solution 4 times for 5 minutes each, and then blocked with normal goat serum in PBS at room temperature for 1 hour. Hybridisation with anti-alpha-2 adrenergic receptor antibody (dilution ratio, 1:1,000; Abcam, Cambridge, UK) was performed at 4℃ for 12 hours. After incubation, Alexa 468-labelled goat anti-rabbit IgG (Invitrogen, Carlsbad, CA, USA) was applied and kept at 4℃ for 12 hours, followed by PBS wash and observed under a fluorescence microscope (GFP for the hair cells and rhodamine for the adrenoreceptors). The expression of the adrenergic receptors was evaluated by overlapping the two images (Fig. 2).
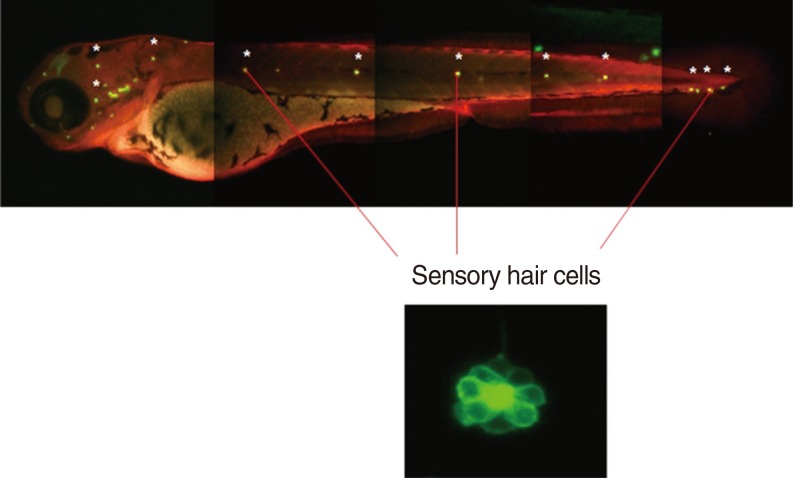
Neuromasts on the lateral side of the zebrafish were observed using fluorescence and confocal microscopes. Cisplatin solution was prepared by mixing the pure form of cisplatin with the embryo culture medium, and the optimal concentration of cisplatin was set as 1 mM, which was the previously reported concentration that induced damages to auditory nerves without inducing mortality in zebrafish [4]. The 5-dpf zebrafish larvae were first pretreated with DEX at concentrations of 0.1, 1, and 10 µM for 150 minutes, washed, and then treated with 1 mM of cisplatin for 6 hours. After treatment, the larvae were washed 3 times and were anaesthetised using tricaine-3-aminobenzoic acid (0.4 g in 100 mL ethyl ester, adjusted to pH 7 by using Tris buffer) for 1 minute, mounted in methylcellulose, and followed by fluorescence imaging. Among the several neuromasts on the lateral side of the zebrafish, 3 ventral neuromasts that were easy to observe (ventral neuromasts of posterior lines P 1, 2, and 3) were selected, and the hair cells of all auditory neuromasts in the 3 selected neuromasts of all zebrafish in the experimental and control groups were calculated by observing under fluorescence and confocal microscopes (LSM5 PASCAL; Carl Zeiss, Jena, Germany) (Fig. 3).
Apoptosis is characterised by DNA fragmentation, which is easily detected by selective staining. A terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) apoptosis staining kit (Roche Molecular Biochemicals, Mannheim, Germany) was used to detect cell death. The 5-dpf larvae were treated with DEX at concentrations of 0.1, 1, or 10 µM for 150 minutes and were treated with 1 mM of cisplatin for 6 hours, followed by fixation and TUNEL staining. The fixed larvae were washed 3 times with PBS containing 0.1%-Tween 20. The enzyme solution and label solution in the TUNEL stain kit were thoroughly mixed and reacted at 37℃ for 1 hour. After the reaction, the cells were washed three times with PBS containing 0.1%-Tween 20 and then examined under a confocal microscope.
We used SPSS ver. 10.0 (SPSS Inc., Chicago, IL, USA) for the statistical analysis. All results, which are expressed as mean±SD. Hair cells counts were evaluated by t-test and one-way analysis of variance was used for multiple comparisons. Significance was accepted for P-values of <0.05.
Neuromasts on the lateral side of the zebrafish were examined under a fluorescence microscope and the number of hair cells in the neuromasts was determined. Cisplatin treatment resulted in a significant decrease in the number of hair cells compared to that in the control. On the other hand, DEX treatment at concentrations of 0.1, 1, and 10 µM significantly attenuated the reduction of hair cells by cisplatin. However, no significant differences in response to dosage and reversal effects of yohimbine were observed (Fig. 4).
Apoptotic cells were marked as light red dots by the TUNEL assay. Comparison of the color intensity between the cisplatin 1 mM group and the DEX-pretreated group under a fluorescent microscope showed that 10 µM DEX significantly decreased the TUNEL reaction and protected the hair cells in the transgenic zebrafish from apoptotic cell death. But we could not find the reversal of DEX effect when we used yohimbin; selective adreno-receptor blocker (Fig. 5).
Preconditioning resulted in a reduction of ischaemic damage that was related to short ischaemia or drug administration prior to long ischaemia. Previous studies have examined the preconditioning effects of anaesthetics on the liver, heart, and kidney [9,10,11], while studies have hardly been performed on the auditory nervous system [12], which is relatively more sensitive to ischaemic damages. Recently, Takeda et al. [13] showed that the induction of an initial short ischaemia in the auditory nerves increased resistance against long ischaemia. Because it is difficult to induce ischaemia during surgery, pharmacologic preconditioning that shows effects similar to that of ischaemic preconditioning is clinically considered as an alternative method. To date, pharmacologic preconditioning effects of the isoflurane, sevoflurane, and desflurane anaesthetics before ischemia have been demonstrated in heart [14,15], although few studies have been conducted on the preconditioning effects of such anaesthetics on the hair cells. Preconditioning effects develop through the activation of mitochondrial adenosine triphosphate-sensitive potassium channels (mito-KATP), increases in the levels of nitric oxide synthase; inhibition of excitotoxic stressor hyperactivity, such as ROS, tumor necrosis factor α, and interleukin 6; or increases in anti-apoptotic factors such as mitogen-activated protein kinase [14,16,17]. In ischaemia caused by noise vibration, when noise vibration continuously occurs, hearing loss develops due to the increased production of ROS in the hair cells, thereby increasing apoptosis. To reduce the progression of hair cell damages to apoptosis, antioxidant activities that prevent intracellular ROS and antioxidants such as glutathione, as well as converting the ROS into less harmful substances should be increased [1,18].
In this study, conditions similar to ischaemia were generated using cisplatin, an anticancer drug known to cause nephrotoxicity, neurotoxicity, and ototoxicity. Ototoxicity of cisplatin is known to occur by decreasing glutathione and antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase) in the hair cells, thereby promoting hypersecretion of ROS [13,19]. The development of ototoxicity by cisplatin is similar to the progression of hair cell damages by ischemia induced by vibration noise [19,20]. On the basis of this similarity, we induced ischaemic damages in the hair cells through cisplatin treatment. Moreover, even though numerous adrenoreceptors are present in zebrafish hair cells, to our knowledge, no studies have been conducted on the distribution of adrenoreceptors in the zebrafish hari cells. We also investigated the occurrence of adrenoreceptors in the zebrafish hair cells.
In present study, we used DEX at a therapeutic range of 0.1-10 µM based on previous studies [21]. DEX is selective alpha-2 adrenoreceptor agonist known to increase antioxidants, and therefore, we assumed that DEX would decrease ROS production via adreno-receptor. As a result, DEX showed preconditioning effect on the hair cells at 0.1, 1, and 10 µM of concentration. However, in this study, the preconditioning effect of DEX was not blocked, though adrenoreceptor was blocked by 100 µM of yohimbine [22] which is selective adreno-receptor blocker. In recent study, a secondary mechanism of DEX through imidazoline receptors has been shown; Dahmani et al. [11] reported that DEX showed preconditioning effects in the hippocampal region through the down-regulation of expression of pERK 1 and 2 in I1-imidazoline receptors instead of alpha-2 adrenoreceptors. The activated imidazoline receptor controlled the production of ROS by downregulating substance like hypoxia-inducible factor. Thus, further studies using knockout zebrafish may facilitate in determining the precise mechanism of action in future.
Nowadays, most head and neck surgery utilises high-frequency drills under general anesthesia, though vibration noise induced hearing loss increases. Ischaemic preconditioning is helpful, but practically difficult to perform during the surgery, thus therapeutic preconditioning by using pharmacologic anaesthetics appears to be a promising alternative approach. This study showed the possibility that DEX imparted preconditioning effects on hair cells and might reduce surgery-related hearing loss.
References
1. Urquhart AC, McIntosh WA, Bodenstein NP. Drill-generated sensorineural hearing loss following mastoid surgery. Laryngoscope. 1992; 6. 102(6):689–692. PMID: 1602918.

2. Farzanegan G, Ghasemi M, Panahi F, Raza M, Alghasi M. Does drill-induced noise have an impact on sensorineural hearing during craniotomy procedure? Br J Neurosurg. 2010; 2. 24(1):40–45. PMID: 20158351.

3. Hong SJ, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of edaravone against cisplatin-induced hair cell damage in zebrafish. Int J Pediatr Otorhinolaryngol. 2013; 6. 77(6):1025–1031. PMID: 23628221.

4. Choi J, Im GJ, Chang J, Chae SW, Lee SH, Kwon SY, et al. Protective effects of apocynin on cisplatin-induced ototoxicity in an auditory cell line and in zebrafish. J Appl Toxicol. 2013; 2. 33(2):125–133. PMID: 22147442.

5. Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, et al. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004; 10. 502(1-2):87–97. PMID: 15464093.
6. Engelhard K, Werner C, Eberspacher E, Bachl M, Blobner M, Hildt E, et al. The effect of the alpha 2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003; 2. 96(2):524–531. PMID: 12538207.
7. Sanders RD, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010; 7. 54(6):710–716. PMID: 20003127.

8. Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, et al. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009; 5. 110(5):1077–1085. PMID: 19352168.

9. Wang J, Meng F, Cottrell JE, Kass IS. The differential effects of volatile anesthetics on electrophysiological and biochemical changes during and recovery after hypoxia in rat hippocampal slice CA1 pyramidal cells. Neuroscience. 2006; 7. 140(3):957–967. PMID: 16580780.

10. Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004; 12. 101(6):1313–1324. PMID: 15564938.

11. Dahmani S, Paris A, Jannier V, Hein L, Rouelle D, Scholz J, et al. Dexmedetomidine increases hippocampal phosphorylated extracellular signal-regulated protein kinase 1 and 2 content by an alpha 2-adrenoceptor-independent mechanism: evidence for the involvement of imidazoline I1 receptors. Anesthesiology. 2008; 3. 108(3):457–466. PMID: 18292683.
12. Steele SL, Yang X, Debiais-Thibaud M, Schwerte T, Pelster B, Ekker M, et al. In vivo and in vitro assessment of cardiac beta-adrenergic receptors in larval zebrafish (Danio rerio). J Exp Biol. 2011; 5. 214(Pt 9):1445–1457. PMID: 21490253.
13. Takeda S, Hata R, Cao F, Yoshida T, Hakuba N, Hato N, et al. Ischemic tolerance in the cochlea. Neurosci Lett. 2009; 10. 462(3):263–266. PMID: 19596048.

14. Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997; 11. 87(5):1182–1190. PMID: 9366471.
15. Kevin LG, Novalija E, Riess ML, Camara AK, Rhodes SS, Stowe DF. Sevoflurane exposure generates superoxide but leads to decreased superoxide during ischemia and reperfusion in isolated hearts. Anesth Analg. 2003; 4. 96(4):949–955. PMID: 12651639.

16. Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000; 5. 28(10):1456–1462. PMID: 10927169.

17. Ljubkovic M, Mio Y, Marinovic J, Stadnicka A, Warltier DC, Bosnjak ZJ, et al. Isoflurane preconditioning uncouples mitochondria and protects against hypoxia-reoxygenation. Am J Physiol Cell Physiol. 2007; 5. 292(5):C1583–C1590. PMID: 17215328.

18. Rybak LP, Kelly T. Ototoxicity: bioprotective mechanisms. Curr Opin Otolaryngol Head Neck Surg. 2003; 10. 11(5):328–333. PMID: 14502062.

19. Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007; 10. 15(5):364–369. PMID: 17823555.

20. Kovacic P, Somanathan R. Ototoxicity and noise trauma: electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: practical medical aspects. Med Hypotheses. 2008; 70(5):914–923. PMID: 17977665.

21. Ruuskanen JO, Peitsaro N, Kaslin JV, Panula P, Scheinin M. Expression and function of alpha-adrenoceptors in zebrafish: drug effects, mRNA and receptor distributions. J Neurochem. 2005; 9. 94(6):1559–1569. PMID: 16000146.
22. Chang Y, Huang X, Liu Z, Han G, Huang L, Xiong YC, et al. Dexmedetomidine inhibits the secretion of high mobility group box 1 from lipopolysaccharide-activated macrophages in vitro. J Surg Res. 2013; 5. 181(2):308–314. PMID: 22939552.
Fig. 1
Experimental protocol. (A) Dexmedetomidine (DEX) preconditioning group. (B) Dexmedetomidine-yohimbine (DEX-YoH) preconditioning group. dpf, days post-fertilization.
Fig. 4
Quantification of zebrafish neuromasts. (A) Hair cell count by fluorescent microscope. (B) The quantification of dexmedetomidine preconditioning effect by hair cell counts. (C) The guantification of yohimbin-blooking effect by cell counts. DEX, dexmedetomidine; YOH, yohimbi.

Fig. 5
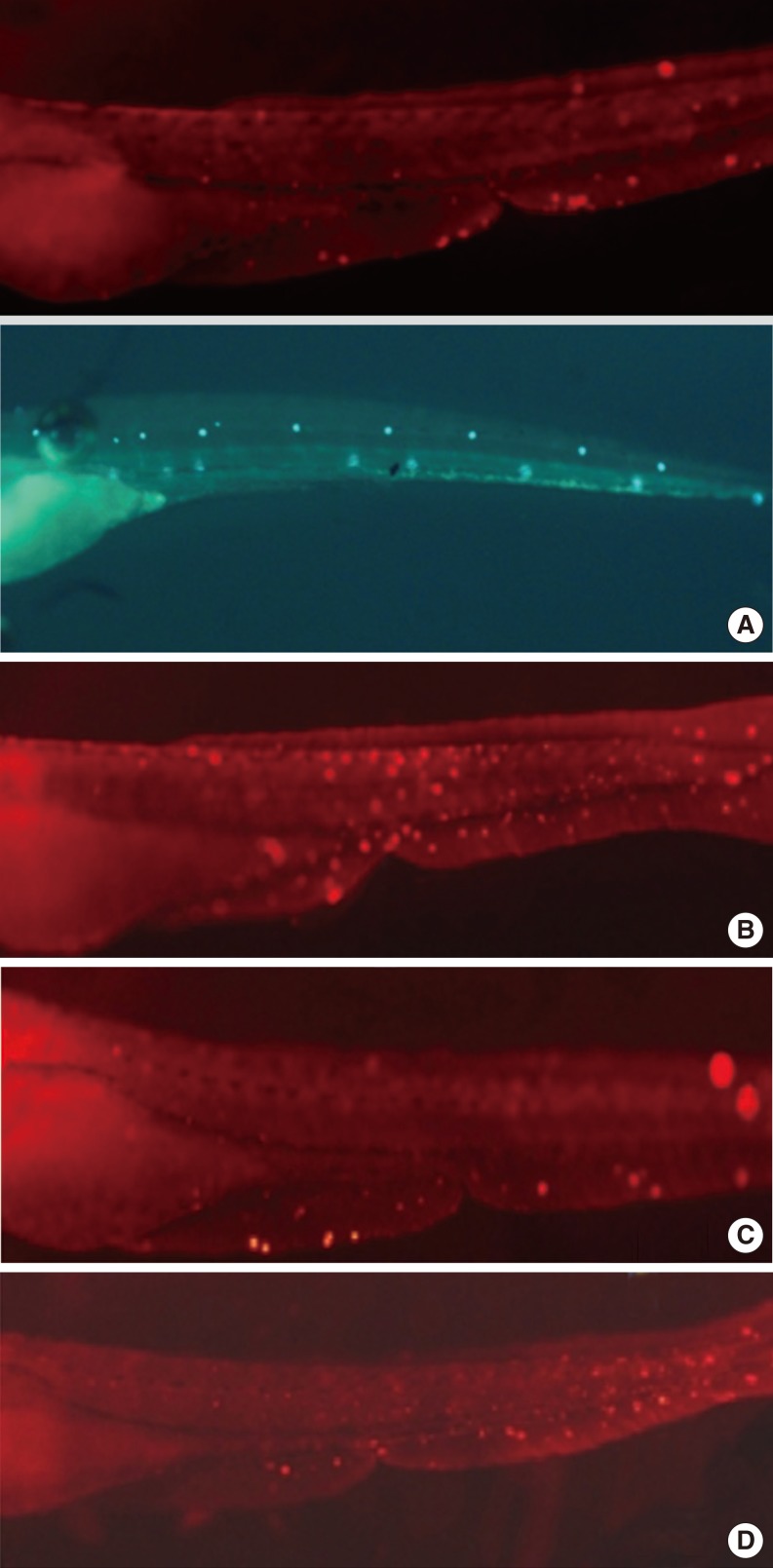
The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay of zebrafishs. Cisplatin-induced apoptotic cells were confirmed by the TUNEL assay. Normal hair cells were marked as green dots. Apoptotic cells were marked as light red dots (arrows) in red-colored fish after TUNEL reaction under a fluorescent microscope. The preconditioning of 10 µM dexmedetomidine significantly decreased the TUNEL reaction and protected the hair cells in the transgenic zebrafish from apoptotic cell death. (A) Control, (B) 1 mM cisplatine (6 hours), (C) 10 µM dexmedetomidine precondition (2.5 hours), 1 mM cisplatine (6 hours), (D) 100 µM yohimbine+10 µM dexmedetomidin precondition (2.5 hours), 1 mM cisplatine (6 hours).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download