Abstract
Primary lung adenoid cystic carcinoma (ACC) is extremely rare and accounts for approximately 0.1%-0.2% of all lung cancers. ACC of the head and neck has generally been regarded as a slow-growing, low-grade malignancy which has a tendency for local recurrence and frequent distant metastasis. When ACC of the lung is identified, physicians must determine whether it represents distant metastasis or a primary lung cancer. Thyroid transcription factor-1 staining is one of the most useful methods to differentiate primary from metastatic lesions in lung cancer. Herein we report a case of metachronous, not synchronous, ACC at the peripheral lung followed by ACC presentation at the base of the tongue, and review of relevant literatures.
Primary lung adenoid cystic carcinoma (ACC) is very rare, accounts for approximately 0.1% to 0.2% of all lung cancers, and only accounts for about 10% of all lung ACCs [1]. Over the last 10 years, approximately 60 cases of primary lung ACC have been reported in the English literatures [2,3,4]. ACC of the head and neck is typically characterized by a slow-growing malignancy which results in approximately 4% to 20% loco-regional recurrence and 20% to 50% distant metastasis. Distant metastasis most commonly occurs in the lung [5,6,7].
When ACC of the lung is identified, it is important to determine whether it represents distant metastasis or primary lung cancer. Thyroid transcription factor-1 (TTF-1) staining is one of the most useful methods to differentiate primary from metastatic lesions in lung cancer [4].
The authors incidentally encountered ACC at the base of the tongue in a patient who was being followed and treated due to primary lung ACC that occurred two years prior. Therefore, we report a case of metachronous, not synchronous, ACC at the peripheral lung followed by ACC presentation at the base of the tongue, with a review of relevant literatures.
The patient, a 54-year-old female, was found to have a mass in the right lower lung (RLL) on chest radiography during a routine health examination in 2010. Thoracoscopic lobectomy of the RLL and lymphadenectomy was performed. The histopathology was reported to be cribriform type ACC. On positron emission tomography-computed tomography (PET-CT) which was performed to evaluate for metastatic disease, no suspicious other primary lesions were identified. Laryngoscopy was performed from nasopharynx to glottis level. There was no suspicious primary lesion. The patient was diagnosed with primary ACC at the peripheral lung.
When the patient was hospitalized after the thoracoscopic lobectomy, cystoscopy was implemented due to hematuria. The patient was diagnosed with bladder cancer and received partial transurethral resection of the bladder. Histopathology demonstrated a non-invasive papillary bladder cancer. During the follow-up period, two years after the primary lung ACC diagnosis, fluorodeoxyglucose uptake on bilateral upper neck lymph nodes were found on PET-CT and the patient was referred to the Department of Otorhinolaryngology-Head and Neck Surgery.
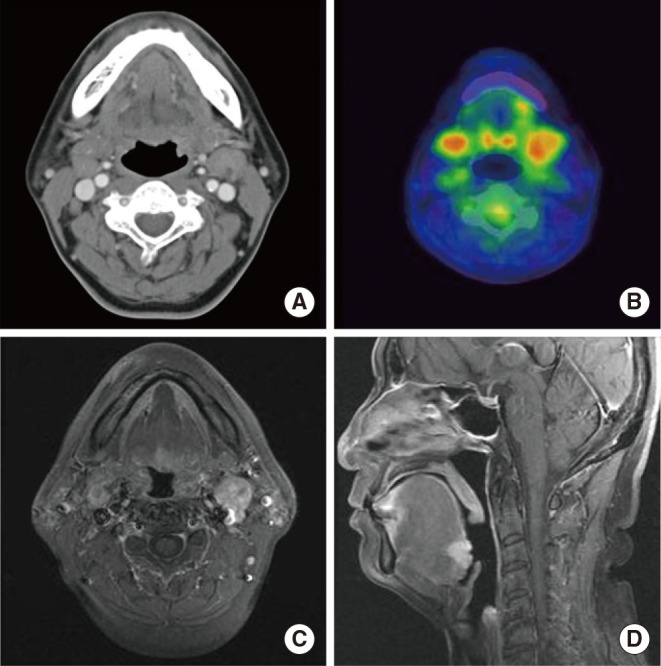
The patient did not report any specific symptoms at the time. Additionally, there were no palpable neck lymph nodes in the bilateral upper neck on physical examination. On laryngoscopy, a small round mass of 1.0 cm was identified in the center of the base of the tongue (Fig. 1). It was firm and fixed on palpation. On CT scan and PET-CT, a lesion was not clearly identified, although enlarged lymph nodes (left, 2.0 cm; right, 1.3 cm) were found at bilateral neck level II areas without contrast enhancement (Fig. 2A, B). Magnetic resonance imaging (MRI) demonstrated a 1.5×1.7 cm mass with T2 contrast enhancement and irregular margins in the center of base of the tongue as well as abnormally enlarged lymph nodes at bilateral neck level II areas (Fig. 2C, D). Biopsy of the lesion was performed and histopathology showed cribriform type ACC. Ultrasound guided fine needle aspiration and cytology was performed on both neck nodes, but only polymorphous lymphoid cells were found.
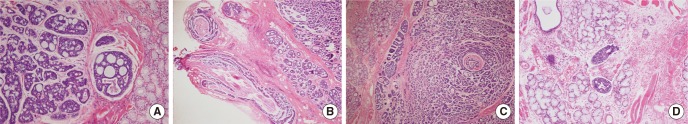
The patient planned to have surgical treatment at the primary site and bilateral necks based upon an American Joint Committee on Cancer (AJCC) cancer stage IVA (T1N2cM0). The primary mass was suspected to extend to the left lingual nerve, and a frozen section was performed to confirm invasion. Cribriform type ACC was identified in histopathologic examination (Fig. 3A). Ligation and excision were performed at the proximal lingual nerve due to invasion (Fig. 3B). The resection margins had sufficient distance for all areas. Perineural invasion (Fig. 3C), lymphatic invasion (Fig. 3D), and single lymph node metastasis less than 1 cm at the level II area without extracapsular spread were also reported. Both enlarged lymph nodes were identified as chronic granulomatous lymphadenopathy, which was considered to be caused by mycobacterium bovis bacillus Calmette-Guérin (BCG) lymphadenitis or other mycobacterial infection. The cancer was stage III (T1N1M0) following surgery. Adjuvant radiation therapy with 5,880 cGy was performed. The patient has continued with follow-up without any recurrence.
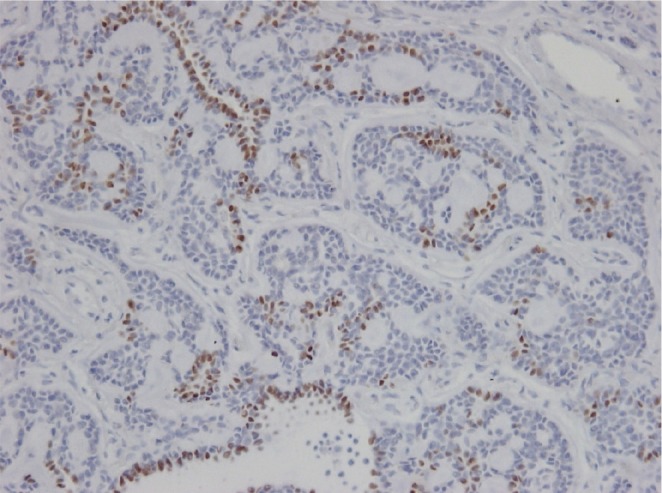
TTF-1 immunohistochemical staining was performed in the previously excised lung specimen to identify whether it was a metastatic or primary lesion. Focal positive (brownish color) staining was identified in TTF-1 staining of the peripheral lung specimen (Fig. 4). It represented a primary cancer in the lung.
Primary lung ACC is extremely rare and represents approximately 0.1% to 0.2% of lung cancer cases [1]. When ACC of the lung is identified, it is important to determine whether the lesion represents distant metastasis or primary lung cancer. In this case, there was no suspicious primary lesion from detailed examination in the head and neck when the lung lesion was initially detected. However, it is possible that a small primary lesion was present in base of the tongue. The small lesion was even difficult to confirm on CT and PET-CT, which were carried out after the lesion had been identified through laryngoscopy. Therefore, in order to differentiate whether the previous lung lesion was a primary lung ACC rather than a metastatic ACC from the base of the tongue, the authors performed additional retrospective TTF-1 immunohistochemical staining of the previous lung resection sample, after treatment of the base of the tongue. TTF-1 is a transcription factor that is expressed in around 60%-70% of thyroid, lung, and pulmonary adenocarcinoma, but not in metastatic lung tumors, except thyroid tumor metastasis. It is expressed in follicle cells of the thyroid and in type II alveolar cells as well as Clara cells of the lung, respectively. TTF-1 is useful to diagnose a primary lung lesion [4]. Thus, TTF-1 staining is one of the most useful methods to differentiate primary and metastatic cancer in the lung.
In the case of a positive result, it is likely to either be primary cancer in the lung or distant metastases of thyroid carcinoma [8]. This staining method also possesses high sensitivity (40%-74%) and specificity (88%-96%) in the diagnosis of a primary lung tumor [4,9]. This case could be also considered as lingual metastasis to the base of the tongue from the lung ACC, even though it is extremely rare. However, lingual metastasis usually was found beneath the normal squamous epithelium of the tongue without dysplasia, thus sparing the tongue mucosa. Additionally, numerous tumorlets were found in the lymphatic vessels, which was further evidence of lymphatic metastasis [10,11,12]. In our case, there was no suspicious sign of lingual metastasis in the base of the tongue specimen.
Several reports represented multiple malignant salivary gland neoplasms. They were most likely synchronously found, different histologic type tumors at the different primary site [13,14,15]. There were a few reports about metachronously found, different histologic type tumors. For example, ACC in the submandibular gland (SMG) followed by mucoepidermoid carcinoma (MEC) in parotid gland 4 years after the treatment of SMG [16], and MEC in palate followed by ACC in the floor of mouth 6 years after the treatment of palate [15]. There was one similar report to our case [13]. In this report, the lung mass were found simultaneously in the patients with proven ACC in the soft palate. It was highly suspicious of metastatic spread to the lung. However, the final diagnosis of the lung was bronchioalveolar carcinoma, then authors demonstrated the value of obtaining tissue diagnosis.
Our case, which was metachronously found, a same histologic type tumor in different sites, is not tied to the above introduced cases. Because ACC is a well-known malignancy which has frequent distant metastasis especially to the lung. If same salivary type tumors, especially ACC, were found both in head and neck and the lung, differentiation primary from metastatic tumor of the lung lesion is important issue.
The incidence of neck lymph node metastases of head and neck ACC is approximately 4%-20%. Only a limited number of studies have been performed with regards to neck lymph node metastases and its management [17]. In the present case, the enlarged lymph nodes at bilateral neck level II areas turned out to be chronic granulomatous lymphadenopathy, which might be induced by either BCG lymphadenitis or mycobacterial infection. BCG irrigation after bladder cancer surgery is increasingly employed as a conservative treatment method [18]. BCG lymphadenitis was reported in some cases after BCG irrigation [19]. However, in this case, other treatments for bladder cancer aside from resection were not performed. In addition, mycobacterium tuberculosis infection was excluded based upon the negative postoperative mycobacterium tuberculosis PCR and culture.
This case described the treatment of metachronous ACC which occurred in the peripheral lung and head and neck during different time periods based upon immunohistochemical staining. To our knowledge, this is the first reported case of metachronously found, a same histologic type tumor, ACC in particular, in the peripheral lung and base of the tongue. However, ACC in the head and neck is susceptible to lung metastasis and can be easily missed if small in size and asymptomatic. For these reasons, detailed head and neck examination including laryngoscopy should be carried out for lung ACC in order to differentiate between primary and metastatic cancer. In particular, bimanual palpation should be performed at the base of the tongue.
When ACC of the lung is identified, it is important to determine whether the lesion represents distant metastasis or primary lung cancer. The otolaryngology examination of head and neck including laryngoscopy and bimanual palpation should be carried out for lung ACC in order to differentiate between primary and metastatic cancer. TTF-1 immunohistochemical staining is one of the most useful methods to differentiate primary from metastatic cancer in the lung. In the case of a positive result, it is likely to be primary cancer.
References
1. Inoue H, Iwashita A, Kanegae H, Higuchi K, Fujinaga Y, Matsumoto I. Peripheral pulmonary adenoid cystic carcinoma with substantial submucosal extension to the proximal bronchus. Thorax. 1991; 2. 46(2):147–148. PMID: 1849668.

2. Kang DY, Yoon YS, Kim HK, Choi YS, Kim K, Shim YM, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer. 2011; 5. 72(2):250–254. PMID: 20884075.

3. Cortes-Telles A, Mendoza-Posada D. Primary adenoid cystic carcinoma of the tracheobronchial tree: a decade-long experience at a health centre in Mexico. Lung India. 2012; 10. 29(4):325–328. PMID: 23243344.

4. Kitada M, Ozawa K, Sato K, Hayashi S, Tokusashi Y, Miyokawa N, et al. Adenoid cystic carcinoma of the peripheral lung: a case report. World J Surg Oncol. 2010; 8. 8:74. PMID: 20796281.

5. Bobbio A, Copelli C, Ampollini L, Bianchi B, Carbognani P, Bettati S, et al. Lung metastasis resection of adenoid cystic carcinoma of salivary glands. Eur J Cardiothorac Surg. 2008; 5. 33(5):790–793. PMID: 18343149.

6. Just PA, Miranda L, Elouaret Y, Meatchi T, Hans S, Badoual C. Classification of salivary gland tumors. Ann Otolaryngol Chir Cervicofac. 2008; 12. 125(6):331–340. PMID: 19036352.
7. Chen AM, Bucci MK, Weinberg V, Garcia J, Quivey JM, Schechter NR, et al. Adenoid cystic carcinoma of the head and neck treated by surgery with or without postoperative radiation therapy: prognostic features of recurrence. Int J Radiat Oncol Biol Phys. 2006; 9. 66(1):152–159. PMID: 16904520.

8. Jagirdar J. Application of immunohistochemistry to the diagnosis of primary and metastatic carcinoma to the lung. Arch Pathol Lab Med. 2008; 3. 132(3):384–396. PMID: 18318581.

9. Johnson H, Cohen C, Fatima N, Duncan D, Siddiqui MT. Thyroid transcription factor 1 and Napsin A double stain: utilizing different vendor antibodies for diagnosing lung adenocarcinoma. Acta Cytol. 2012; 56(6):596–602. PMID: 23207437.

10. Terashima T, Matsuzaki T, Kawada I, Nishida J, Tanaka Y, Morishita T, et al. Tongue metastasis as an initial presentation of a lung cancer. Intern Med. 2004; 8. 43(8):727–730. PMID: 15468975.

11. Yoshitomi I, Kawasaki G, Mizuno A, Nishikido M, Hayashi T, Fujita S, et al. Lingual metastasis as an initial presentation of renal cell carcinoma. Med Oncol. 2011; 12. 28(4):1389–1394. PMID: 20567942.

12. Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity: pathogenesis and analysis of 673 cases. Oral Oncol. 2008; 8. 44(8):743–752. PMID: 18061527.
13. Hadfield PJ, Fisher C, Archer DJ. Adenoid cystic carcinoma of the maxilla: the value of histopathology in diagnosing a second primary. J Laryngol Otol. 1996; 5. 110(5):503–506. PMID: 8762331.
14. Bab IA, Ulmansky M. Simultaneously occurring salivary gland tumors of different types. J Oral Surg. 1979; 11. 37(11):826–828. PMID: 226666.
15. Whitt JC, Schafer DR, Callihan MD. Multiple malignant salivary gland neoplasms: mucoepidermoid carcinoma of palate and adenoid cystic carcinoma of floor of mouth. Head Neck Pathol. 2008; 3. 2(1):41–48. PMID: 20614341.

16. Hosni A, Fisher C, Rhys-Evans P. Two malignant salivary gland tumours of different type in one patient. J Laryngol Otol. 1994; 9. 108(9):798–800. PMID: 7964150.

17. Min R, Siyi L, Wenjun Y, Ow A, Lizheng W, Minjun D, et al. Salivary gland adenoid cystic carcinoma with cervical lymph node metastasis: a preliminary study of 62 cases. Int J Oral Maxillofac Surg. 2012; 8. 41(8):952–957. PMID: 22647764.

18. Askeland EJ, Newton MR, O'Donnell MA, Luo Y. Bladder cancer immunotherapy: BCG and beyond. Adv Urol. 2012; 2012:181987. PMID: 22778725.

19. Geldmacher H, Taube C, Markert U, Kirsten DK. Nearly fatal complications of cervical lymphadenitis following BCG immunotherapy for superficial bladder cancer. Respiration. 2001; 68(4):420–421. PMID: 11464093.

Fig. 1
Fibroscopic finding at the base of the tongue. A 1.0-cm, round, firm, and fixed lesion was identified.
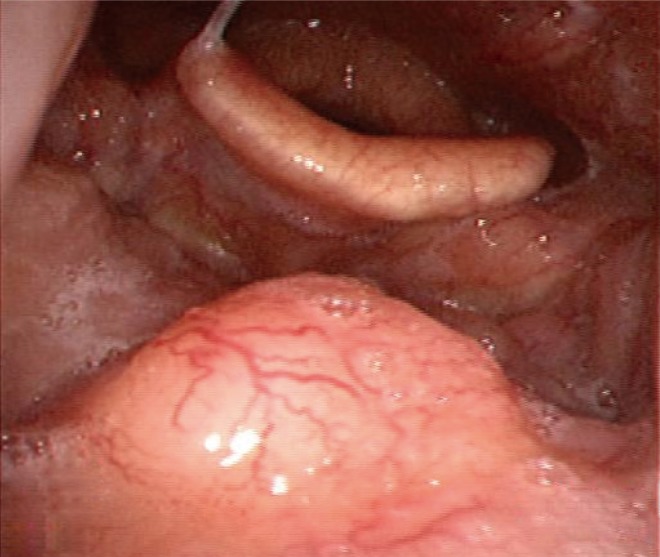
Fig. 2
Image findings. (A) Neck computed tomography (CT) contrast-enhanced view (axial): enlarged lymph nodes are identified at bilateral level II neck areas (left, 2.0 cm; right, 1.3 cm). (B) Positron emission tomography-CT: enlarged lymph nodes have increased fluorodeoxyglucose uptake. (C, D) Neck magnetic resonance imaging. T1 gadolinium enhanced view: enlarged lymph nodes at the level II neck area (2-3 cm) were identified (axial) and a 1.5×1.7 cm mass with irregular margins was observed at the base of the tongue (sagittal).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download